Chair, DIA Board of Directors

DIA Global Chief Executive
proved to be our most productive and impactful year in many decades. Thank you for your commitment to our mission to apply our global, neutral forum to share and generate knowledge accelerating healthcare innovation for patients worldwide.
Our work to capture and openly share this knowledge exchange has led to advances in digitization. We saw our newly created tool, DIA NOW, add tremendous value to R&D organizations around the world. Finally, our work to advance the capacity and capability of healthcare professionals globally triggered an expansion in our DIA Learning portfolio, and our mobile-first digital approach allowed the knowledge of subject-matter experts to get directly into the hands of people seeking to expand their skills, no matter where they are around the globe.
For our board and staff, the most rewarding observation is that we know that asking the right questions, in the right environments, with the most expert voices sharing in the debate, stimulates engagement and participation, and has a real impact on the healthcare of people. In 2018, we saw record participation in DIA meetings and learning events, and we are increasing our efforts to foster more robust Communities and membership. Our publications are seeing expanding readership across the board, and the interest in participating and contributing to our research is very strong.
Our vision—to be your essential partner in catalyzing knowledge creation to accelerate healthcare product development—is being realized and will only grow more deeply in the coming years. We look forward to continuing the work we do as a community of tens of thousands of life science professionals around the globe that comprise DIA and collectively make a difference in the lives of patients.
Sincerely,



are a global association that mobilizes life science professionals from around the globe and across all areas of expertise to engage with patients, peers, and other experts in a neutral environment on the issues of today and the possibilities of tomorrow.

Engaging Patients
s the call for meaningful and impactful patient engagement grows stronger, so does the push for an increasingly empirical approach to address the topic. In 2018, we continued to drive the movement towards evidence-based patient centricity and engagement across the entire healthcare ecosystem.
- We released new findings of our 2016 multi-phase research study on patient-centric initiatives in drug development conducted in partnership with Tufts University. Published in DIA’s peer-reviewed journal Therapeutic Innovation & Regulatory Science (TIRS), the article Measuring the Impact of Patient Engagement and Patient Centricity in Clinical Research and Development describes patient engagement metrics and their use in determining return on engagement.
- Kick-off of Phase II of the study, called “Assessing Patient Centricity Preparedness, Capability, Experience, and Impact.”
- Five key DIA events provided a platform for patients and other stakeholders to share fresh, multi-faceted insights on meaningful patient engagement.
- We incorporated patient engagement concepts for industry into the DIA Learning portfolio with the release of the new eLearning module Introduction to Patient Engagement in Drug Development. Also this year, two new drug safety courses were released and included specific concepts on incorporating patient perspectives into these aspects of drug development and lifecycle management: Benefit-Risk Assessment and Management Across the Lifecycle, and Safety Risk Communication for Medical Products.
- The DIA Patient Engagement Community (PEC) continued to expand its membership as well as its global presence with the start-up of a new chapter in Japan.
Why we care: At DIA we have long recognized the important role patients play in shaping the care they need. Involving patients in therapeutic product R&D and developing methods to characterize the impact of patient feedback and return on engagement helps drive the development of better healthcare products and therapies that deliver meaningful health outcomes.
Advancing Therapies from Bench to Bedside
n 2018, DIA continued to provide a unique forum for advancing the field of translational science by collaborating and exchanging fresh insights with leading health authorities, industry, regulators, patients, and academics.
- Together with the Tufts Center for the Study of Drug Development we started a PharmaTech research study to identify the most promising and analytical digital platforms currently used or planned in healthcare. The first detailed outcomes have been submitted as a peer-reviewed article.
- We continued our successful collaboration with the National Center for Advancing Translational Sciences (NIH-NCATS) in 2018 to address two important topics in clinical research: tissue chips and rare diseases.
- A working group of experts from across the healthcare sector explored how tissue chips and clinical trials on chips (CToCs) could best be used to move the development of rare disease therapies forward.
- Industry and government representatives took on rare diseases in a workshop where discussions centered on how to bring advocacy organization together with industry and government to advance rare disease clinical research.
- For the fourth time since its inception in 2015, the Drug Discovery Innovation (DDI) Conference in Suzhou, China brought together representatives from internationally renowned pharmaceutical companies, local start-ups, and academia.
- Members discussed cutting-edge topics within the field of translational science in DIA meetings around the world, numerous publications and podcast, and our DIA Communities.
Advancing Regulatory Science
ith our ability to foster conversations among regulators, industry, and patients we successfully position ourselves at the intersection of cutting-edge scientific advances, current international regulation, and safe treatment access for patients. As in previous years, our activities this year supported the global evolution of regulatory science to increase efficiency through regulatory convergence and data and submission standards.
Following recommendations from the DIA Council of Regulators (COR), we continued to address current regulatory challenges, including ICH training needs, generic drug development, and the use of Artificial Intelligence (AI) and Real World Data (RWD) in regulatory decision making as well as other global issues.

- As Authorized Training Partner of the ICH (International Council for Harmonization), we continued to offer ICH-approved courses in alignment with the latest ICH guidelines.
- The Duke-NUS Centre of Regulatory Excellence (CoRE) and DIA worked together to organize the inaugural DIA-CoRE Conference in Singapore.
- We hosted our first 2018 Real World Evidence (RWE) Conference in San Francisco to explore applications of RWE and describe novel ways stakeholders are leveraging RWE to advance healthcare knowledge and decision-making.
- We provided several platforms for regulators and industry representatives to collaborate more closely on new technologies, such as AI and gene editing, that are about to enter the regulatory space.
- In Japan, we co-hosted the International Coalition of Medicines Regulatory Authorities (ICMRA) as part of the 15th DIA Japan Annual Meeting.
- At our second USFDA-EMA-CDSCO-DIA Multicenter GCP Workshop in India, stakeholders discussed General Clinical Practice (GCP) guidances and polices.
- In collaboration with Office of Generic Drugs at FDA we built our first Complex Drug-Device Generic Combination Products Meeting related to combination products and complex generics, such as inhalation drugs.
Better Value, Quicker Access
n 2018, we focused our activities around three key areas to advance thought leadership in the value and access space: (1) sustainability of healthcare funding, (2) defining the concept of unmet medical need, and (3) supporting topics identified in the EMA-EUnetHTA (European Network for Health Technology Assessment) work plan to facilitate public discussions. Each project was proposed by DIA members and stakeholder groups as initiatives in which DIA should take a leading role.
- In partnership with the Boston Consulting Group (BCG) Market Access Roundtable, we further formalized the Sustainability of Healthcare Funding initiative into a project with leading academics to spearhead research streams on payment models, data and data infrastructure, and patient and cross-stakeholder collaboration.
- The European payer and regulator community initiated a working group to define unmet medical need and invited DIA to lead the discussion. The initiative was extended to trans-Atlantic stakeholders with the ambition to globalize some of the identified criteria.
- Organized for the second time in Europe, the Value, Access, and Regulatory Strategy workshop addressed areas that market access and regulatory strategies have in common. The discussion ranged from EMA-EUnetHTA Parallel Consultation (the process where scientific advice is sought from EMA and HTA bodies simultaneously) and increasing cooperation in health technology assessment to the various opportunities that digital health can offer and the use of real world evidence.
- DIA Japan hosted the 1st DIA Health Economics and Outcomes Research (HEOR) Workshop to discuss the progress in HEOR area.
Why we care: There is an increasing need for non-political and non-commercial platforms to discuss optimization of patients’ access to affordable, high-quality medicines. With the incoming wave of modern technologies, such as AI and advanced gene editing, it’s becoming increasingly important to reevaluate the suitability of most value frameworks and payment models. As new technologies are entering the market, it’s time to develop better solutions to enable faster access to treatments that would benefit not only the patient but also the healthcare systems. At DIA, we are perfectly positioned to connect thought leaders to accelerate this change.
Learning with DIA
tilizing a blended curriculum, DIA delivers best-in-class education to thousands of professionals worldwide looking to advance their careers by improving their skills. In 2018, DIA offered four new face-to-face courses and five new online learning programs in addition to the diverse set of online, on-site, and blended courses already available.
Why we care: At every stage of their career, healthcare professionals have to stay abreast of critical issues spanning the healthcare product lifecycle. Now more than ever, it is critical to offer professional training that is current, effective, relevant, and trusted.
Making Connections
cross corporate, regulatory, and international boundaries, DIA Communities and Working Groups serve as easily accessible hubs for stimulating peer-to-peer discussions, professional networking opportunities, lively idea exchanges, and high-quality content created by our members. Younger professionals and students can advance their careers and develop new skills by joining our new “Leader of Tomorrow” program and competing in the “Leader of Tomorrow” challenge.
Why this matters: DIA Communities and our Leader of Tomorrow program offer DIA members at different career levels exclusive access to global conversations around healthcare. Community members work together to speed innovation in healthcare product development, producing resources such as tools, case studies, and best practices that benefit the entire healthcare ecosystem.

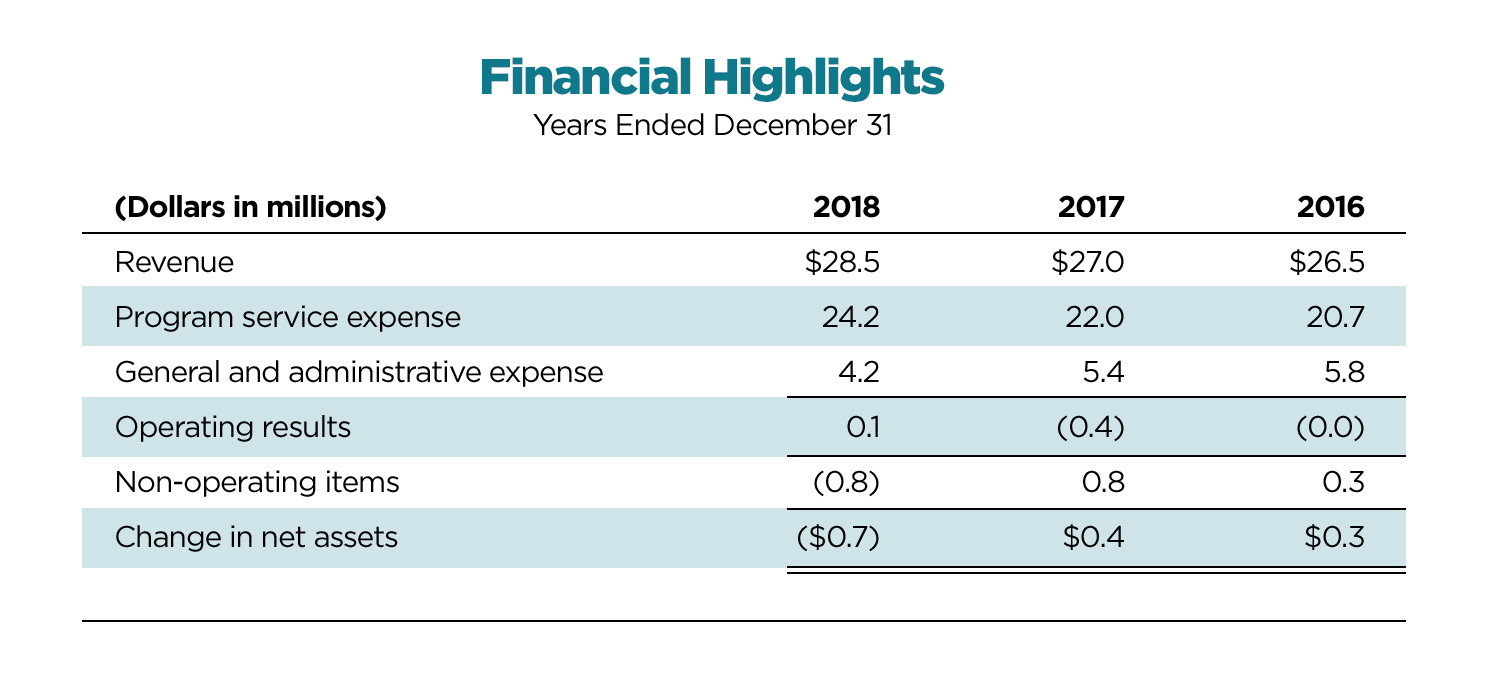
DIA Financials
or the year ending December 2018, DIA delivered a contribution to operating financial results, as well as a positive change in net assets.