compelling but underappreciated hallmark of cancer is its pattern of growth. Cancers do not grow exponentially as they would were growth cellular autonomous. Rather, their growth follows sigmoid (“S” shaped) curves. As described in our article published in the August 2021 issue of Global Forum, this fact has been shown to be helpful in the design of effective anticancer chemotherapy regimens, as illustrated by the treatment of primary breast cancer. But what is the etiology of sigmoid growth? And can explorations of this question be used to improve the application of novel anticancer therapeutics, especially the antibody-drug conjugates (ADCs), which differ mechanistically from chemotherapy in several key respects?
In 2005, Andrew Minn and colleagues, working in the laboratory of Joan Massagué, published the observation that a derived cancer cell line selected to cause a higher incidence of lung metastases grew faster in the implantation site (mammary fat pad) than the parental, low-metastasizing cell line. This increased growth rate was not associated with a higher percentage of dividing cells as measured by the Ki67 fraction. How can a mass grow faster and yet not have a higher growth fraction?
A possible solution to this mystery was proposed in 2006: Perhaps, in addition to traveling to distant sites like the lung, metastasizing cells could return to the site of origin, in this case the mammary fat pad, and in recolonizing that primary location create many foci of growth, which would give an increased total growth rate despite similar growth rates of each individual focus. This phenomenon, called self-seeding, was subsequently tested and validated by Mi Young Kim and colleagues in Massagué’s laboratory. More recently, Jonathan Weissman and colleagues have observed the phenomenon in human tumor xenografts as assessed by single-cell DNA sequencing.
Self-Seeding and the Tumor Microenvironment
Self-seeding has many potential clinical implications. Its application to tumor growth kinetics is as follows: If cancers grow by seeding themselves, then the primary site of growth must be the contact space between the cancer and its surroundings, now commonly called the tumor microenvironment. In other words, cancers grow at least partially from the outside in rather than just from the inside out. Outside implies geometry. For example, were the tumor mass a pure sphere, its outside would be proportional to the square of its diameter while the inside would be proportional to the cube of its diameter. Since, as the mass gets bigger, the square of the diameter increases more slowly than the cube of the diameter, the outside-to-inside ratio gets smaller with increasing tumor size. Hence, the relative rate of outside-in growth decreases with increasing size, resulting in sigmoid growth. Of course, cancerous masses are not simple spheres, so we are not talking about exponents like two and three. But, in geometric terms, if the outside space has a dimension (an exponent) less than that of the inside, then the basic geometric concept is still applicable.
Sigmoid growth, therefore, is in essence a manifestation of the biology of cancer cells in relationship to their microenvironment. Cancers that are highly dependent on their relationship to their microenvironment may not only grow faster than those that are more independent, but because of the geometric principles above they may exhaust that stimulation faster, thereby providing an explanation for the relationship between Gompertzian parameters discussed in our previous article.
Self-seeds not only induce new blood vessel growth but also increase tumor infiltration with leukocytes. More recent work has shown that tumor-infiltrating leukocytes may themselves harbor oncogenic mutations, the full impact of which remains to be defined. Moreover, induced changes in the microenvironment are essential for the successful colonization by the seeds. It is a reasonable hypothesis, therefore, that antibody-drug conjugates, which have shown remarkable anticancer effects, are successful partially because they exploit these geometric considerations by adversely influencing microenvironmental components as well as by killing cancer cells.
ADC Properties and Mechanisms
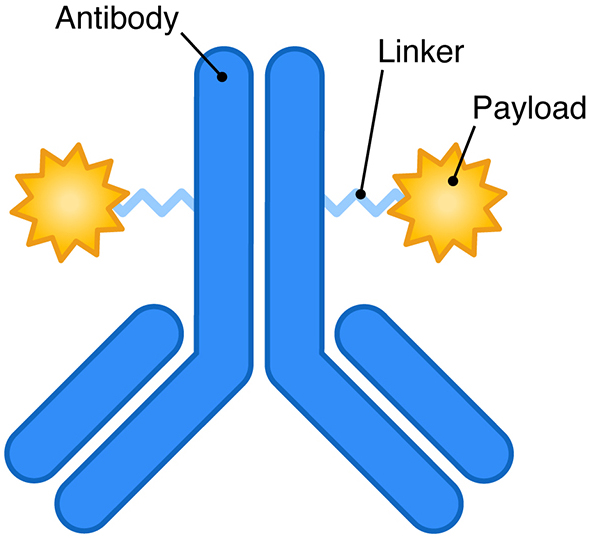
All ADCs are composed of three obligatory components: a monoclonal antibody, a cytotoxic payload, and a linker that connects the two (Figure 1). The antibody component is typically directed at a specific protein antigen on the cancer cell. The idea here is to deliver a highly potent cytotoxic payload to the cancer cells in a targeted fashion. Accordingly, ADCs have been compared to “targeted missiles” or “Trojan horses” because of their putative ability to selectively attach to and thereby damage cancer cells. However, the mechanism of action of ADCs is far more complex. No linker is perfectly stable. Instability allows for the release of payload both in the peripheral circulation and in the tumor microenvironment prior to engagement with tumor cells.
Exploiting this property, some next-generation ADCs are specifically designed to release lipid-soluble payloads after they are internalized inside cancer cells. These can diffuse across cell membranes and exert their cytotoxic effects on neighboring cells, including cells that do not express the antigen target. This so-called bystander-effect is indeed thought to be essential to the activity of certain ADCs, especially in tumors with low or heterogeneous expression of the target antigen (Figure 2).
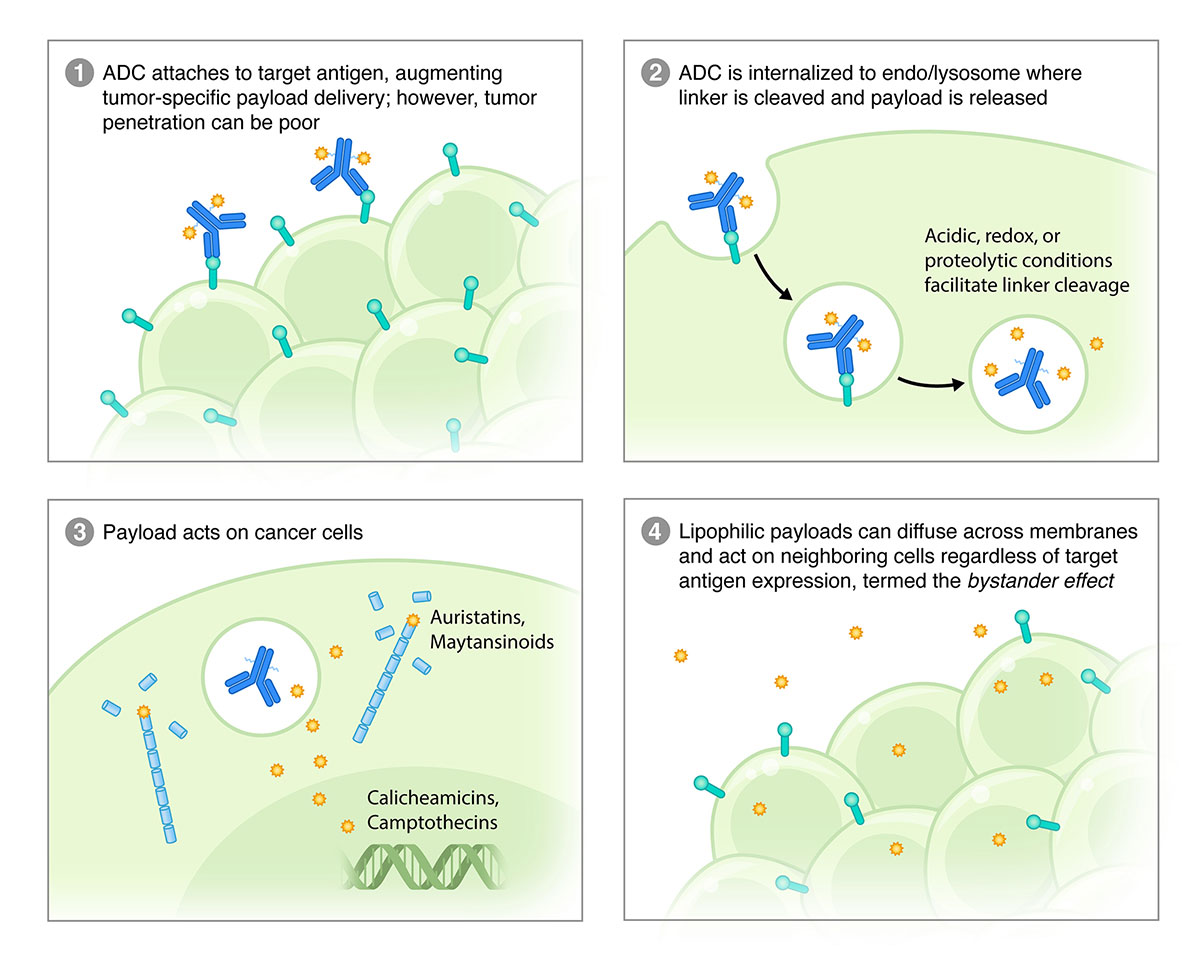
Precisely targeted therapies are particularly effective against cancers with homogenous expression of the drug target. However, precision targeting is a liability when dealing with cancers that are heterogeneous in that regard. For this reason, ADCs present the possibility of being the best of both worlds: highly targeted and capable of killing cells in the vicinity that lack the precise target. Vulnerable in this respect are stromal cells—leukocytes, endothelial cells, fibroblasts—in addition to targetless cancer cells. Furthermore, indiscriminate killing could alter the microenvironment in productive ways. For example, ADCs in combination with cytotoxic drugs have been shown to augment antitumor immunity via increased recruitment of tumor-infiltrating lymphocytes and promotion of antibody-dependent cellular toxicity.
The pharmacokinetics and pharmacodynamics of ADCs are nascent fields of study. However, it is already clear that, while ADCs combine antibodies and a toxic payload, there are major differences between ADCs and unlinked monoclonal antibodies and cytotoxic drugs. Different ADCs can exhibit markedly different behavior owing to a variation in antibody-antigen interactions, stability of the linker and cleavage mechanism, and payload properties. ADCs are large and therefore diffuse relatively slowly into large tumors. ADCs comprised of antibodies with strong affinity for a highly expressed targeted antigen may be “spent” on the surface of tumorous masses before they are able to penetrate them, which is called the binding-site barrier effect. Although different agents differ in hepatic clearance, ADCs often clear faster than unattached monoclonal antibodies.
Hence, there are many opportunities for development by laboratory and clinical research. Among the issues to be addressed are optimal dosing schedules. Here growth kinetic thinking could prove helpful. For example, at present no approved ADC is routinely given with granulocyte growth factor support despite their ability to induce treatment-limiting bone marrow suppression. The principles of dose density should be just as applicable here as in more conventional cytotoxic chemotherapy, and perhaps more so because of the relationships between surface area and volume discussed above. By geometry, small tumors have greater magnitudes of tumor-microenvironment interface than if they were larger. And some (i.e., the faster growing ones that plateau at lower sizes but increase total body burden of cancer by metastasizing, including to the organ of origin) may be more dependent on that microenvironment. Hence, ADCs may be ideally suited to increase cell kill as the tumor shrinks under the influence of therapy.
This effect could be amplified by the kinetics of antigen concentration. For ADCs the tumor-specific dose of cytotoxic payload delivery, and hence the anticancer activity, is proportional to the quantity of the target antigen within a tumor as a function of the tumor volume. This was shown for ADCs having HER2—a prototypic target in many breast cancers: Tumors with high and homogeneous HER2 expression are more sensitive to HER2-targeted ADCs. But target expression is not static over time, especially under the pressure of ADC treatment. Indeed, target antigen downregulation or loss is thought to be an important mechanism of resistance to ADCs.
This raises the possibility of an induction-intensification approach. Here, the tumor volume is first reduced by a therapy other than an ADC, reserving the application of the ADC for consolidation at a time when both the tumor’s surface-to-volume ratio and antigen concentration are prime for sensitivity to an ADC. Since untargeted dose-dense sequential therapy has already been shown to increase cure rates in primary breast cancer, an employment of the concept in a targeted fashion might be even more effective. Moreover, these considerations are relevant for biomarker discovery in that the threshold level and spatial distribution of a tumor-specific antigen to optimize ADC activity is yet unknown.
So, while much work remains to be accomplished, the implications of the many advantages of ADCs as a class of therapeutic agents are profound. Their major asset—high cytotoxic payload delivery in a molecularly specific manner—are clear. But the ways in which this influences the tumor microenvironment and how it could lead to cancer cell eradication demand elucidation. Additionally, how to optimize dosing, scheduling, and patient-selection strategies beg for further development. That these agents have empirically demonstrated impressive activity so far should motivate principle-driven research and innovation to improve results. Kinetics—pharmacokinetics, growth kinetics, and geometric kinetics—should be brought to bear in this quest. The next decades will no doubt bring many advances, fueled by creativity and rewarded by therapeutic progress.
References available upon request.

