Pharmacovigilance Lessons from a Model Pandemic Safety Oversight Response
Gates Foundation
he African Union Smart Safety Surveillance (AU-3S) program represents a transformative initiative in strengthening pharmacovigilance (PV) systems across Africa. Designed to address the continent’s unique challenges, this ambitious 10-year program, funded by the Gates Foundation, aims to create a centralized PV infrastructure that enhances confidence in the safety of medical products for African regulators and patients alike.
The need for rapid safety surveillance is also particularly urgent in the context of pandemics and fast-tracked approvals, such as those seen during COVID-19. Digital health tools have demonstrated great potential in increasing adverse event (AE) reporting, but challenges remain in terms of interoperability, adaptability, and effective scaling. Moreover, the right technical capacity and capability to detect and act on potential signals must be in place. Addressing these challenges requires end-to-end strategic collaborations, innovative technologies, and sustainable solutions.
The AU-3S Program pilot was critical in enabling the safe launch of COVID-19 vaccines across 35% of the African population during the pandemic and built on earlier successful collaborations with WHO and UK MHRA1. Employing the Smart Safety Surveillance (3S) methodology, AU-3S focuses on strengthening drug safety (pharmacovigilance, or PV) systems for priority diseases through scalable and sustainable interventions.
Responding to In- and Cross-Country Needs
In the midst of the COVID-19 pandemic, regulators faced a dilemma: The imminent arrival of vaccines that had not been tested in their populations, coupled with strong governmental and public pressure to approve them for regulated use in their respective jurisdictions. In response, AU-3S partnered with five pilot countries (Ethiopia, Ghana, Nigeria, South Africa, and later Kenya), with the UK MHRA as a technical partner and the US FDA and the WHO African Regional Office (AFRO) as advisors, to fast-track the deployment of both in-country and cross-country end-to-end safety solutions. The countries were strategically chosen for their demographic significance, existing PV capabilities, and as entry points to the continent for the first COVID vaccines.
Initial assessments revealed several shared gaps across these countries, including limited awareness of AE reporting among healthcare workers and patients, reliance on outdated paper-based forms, silos between the NRAs (National Regulatory Authorities) and public health programs, and insufficient resources for PV activities.
To address these issues, AU-3S introduced the Med Safety app, a globally recognized tool for both provider and patient AE reporting. Customized reporting forms based on the WHO AEFI reporting template were incorporated into the app, reducing training needs and streamlining AE submission during the rollout. Training modules covering all aspects of safety surveillance, as well as dedicated funding, were made available to countries, further strengthening their capacity to detect, analyze, and respond to safety signals.
At the continental level, AU-3S established the Data Integration and Signal Detection (DISD) system, a pioneering platform for cross-country safety data integration. Hosted within MHRA’s Sentinel system during its interim phase, DISD enabled near-real-time signal detection and analysis. It marked a significant milestone as the first African-owned pan-African safety database, allowing pooled data analysis across multiple countries. It also ensured that all cases were simultaneously submitted to the WHO global safety database, VigiBase.
The program also formed a Joint Signal Management (JSM) Group comprising regulatory and safety experts from the participating countries. This group validated and assessed cross-country signals, making evidence-based safety recommendations, and reinforcing national PV efforts as needed.
The innovative in- and cross-country model can be summarized as follows:

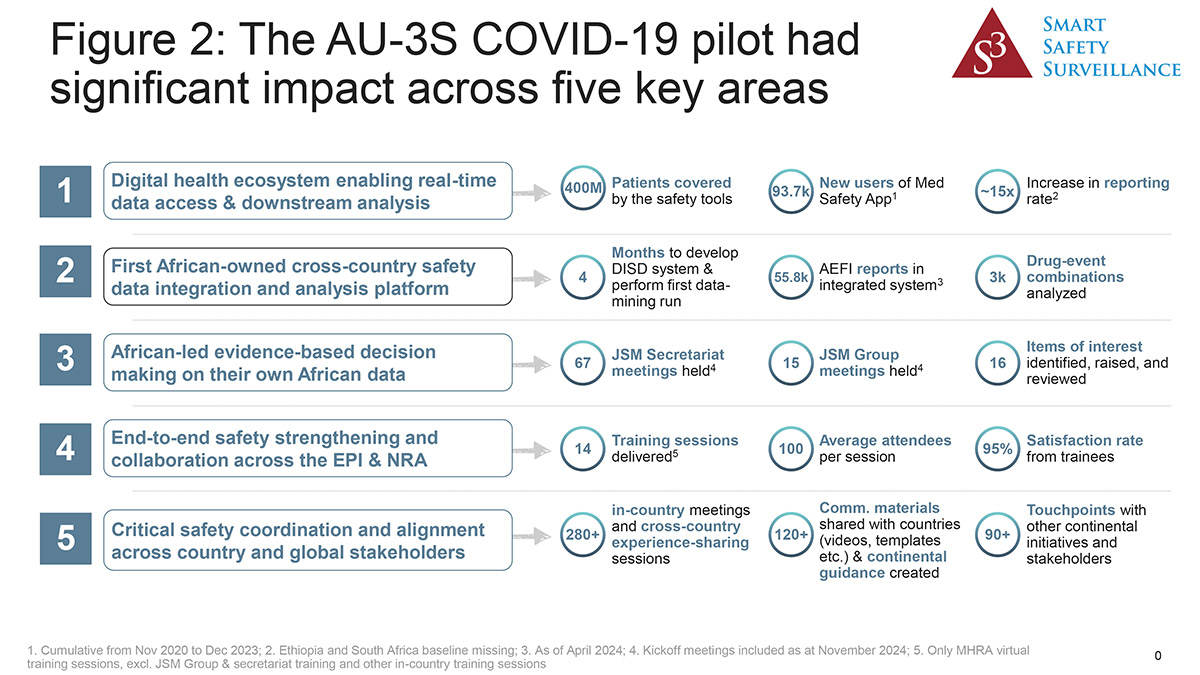
As a result of these efforts, African regulators were able to use African data to prove the safety of COVID-19 vaccines in African patients. As there were scant African data available from clinical trials and given that certain comorbidities are present in higher prevalences in Africa than in other parts of the world, this reassurance was welcomed.
Evolution of AU-3S Post-Pandemic: Continental Expansion Roadmap
The success of the AU-3S COVID-19 pilot has laid a strong foundation for the expansion of AU-3S to further serve continental safety needs. The program has developed this strategic roadmap to scale its reach to include additional priority medicinal products, member countries, and technologies:
- Country Expansion: By December 2024, the program had grown to include 12 member states, adding DRC, Egypt, Mozambique, Rwanda, Senegal, Tanzania, and Uganda. Selection criteria for new members emphasized the presence of functional safety systems, geographic representation, and language diversity. Plans are underway to onboard additional countries in 2025.
- Product Expansion: Beyond COVID-19 vaccines, AU-3S now monitors critical products for AU priority diseases like HIV/AIDS, tuberculosis, malaria, and maternal health. Emerging vaccines for Lassa fever, respiratory syncytial virus (RSV), and Group B Streptococcus are also planned as well as vaccines and therapeutics for emergency situations (e.g., mpox).
- Technological Advancements: The interim DISD system has transitioned to a state-of-the-art platform called Safety Connect, a critical step toward the creation of AfriVigilance. This is the planned continental safety database for Africa to be eventually hosted by the African Medicines Agency3. Safety Connect integrates a vigilance receiving hub, safety database, and signal detection tool, enabling a scalable and sustainable technology solution.
- AU-PRAC: Another pivotal development is the transition of the JSM Group to the African Union Pharmacovigilance Risk Assessment Committee (AU-PRAC). This committee will oversee signal detection, analysis, and safety recommendations (supported by two specialized technical hubs for vaccines and medicines) for the expanded list of priority products. Comprised of signal management experts from the African Member States, and other experts, it will also act as an expert advisory group to the newly formed AMA (African Medicines Agency) Pharmacovigilance Technical Committee.
Success Factors and Looking Forward
The importance of the assets of AU-3S in shaping the future PV strategy for the African continent has been acknowledged, and the AU Assembly in February 2024 formalized its request for AU-3S to build a framework for the future PV monitoring remit of AMA.
Several key factors have contributed to this success:
- Collaboration: Strong partnerships between NRAs, public health programs, and technical partners were instrumental in increasing AE reporting rates and enabling end-to-end safety monitoring and analysis.
- Quality Management: Interoperable systems facilitated seamless data sharing and analysis across healthcare systems, enhancing the ability to detect and address safety concerns promptly.
- Faster Risk Assessment: Access to a larger pool of integrated cross-country data, and the right technical support to analyze those data, allowed for quicker identification of potential risks during the pandemic.
- Improved Data Sharing: Standardized processes and comprehensive data sets streamlined AE reporting to WHO VigiBase, further adding to the body of safety data available globally on COVID vaccines.
Empowering Regulators, Increasing Patient Confidence
AU-3S has established itself as a cornerstone of pharmacovigilance and patient safety in Africa. By leveraging digital tools like the Med Safety app and implementing innovative platforms such as the DISD, the program has set a new standard for safety surveillance on the continent. Its success during the COVID-19 vaccine rollout demonstrates its scalability and adaptability, offering a model for pandemic preparedness and beyond.
Through regional collaboration, capacity building, and the integration of cross-country safety data, AU-3S empowered African regulators and increased patient confidence that these regulators can safeguard their health, even as products are developed and made available through accelerated pathways. The program’s approach can position Africa as a leader in global pharmacovigilance while addressing the unique needs of its diverse populations.
As AU-3S evolves, its vision for an end-to-end PV ecosystem continues to inspire confidence and is further enabled by the mandate to form the future PV monitoring remit of AMA. The program will ensure that Africa is better prepared to tackle future health challenges, helping to safeguard millions of lives across the continent and serving as a blueprint for LMICs worldwide, proving that innovation and collaboration can transform public health outcomes.

