Special Section: Pharmacovigilance
mart Safety Surveillance (3S) is a product-focused, principles-based approach to building functional LMIC (low- and middle-income countries) pharmacovigilance (PV) systems that has been validated across multiple products and geographies. The lessons learned and key success factors enabling this work have been shared with interested country regulators and public health decision makers tasked with protecting the safety of their populations, funders, and multilateral organizations partnering with these countries, and to product developers developing global product launch plans.
What is the Need?
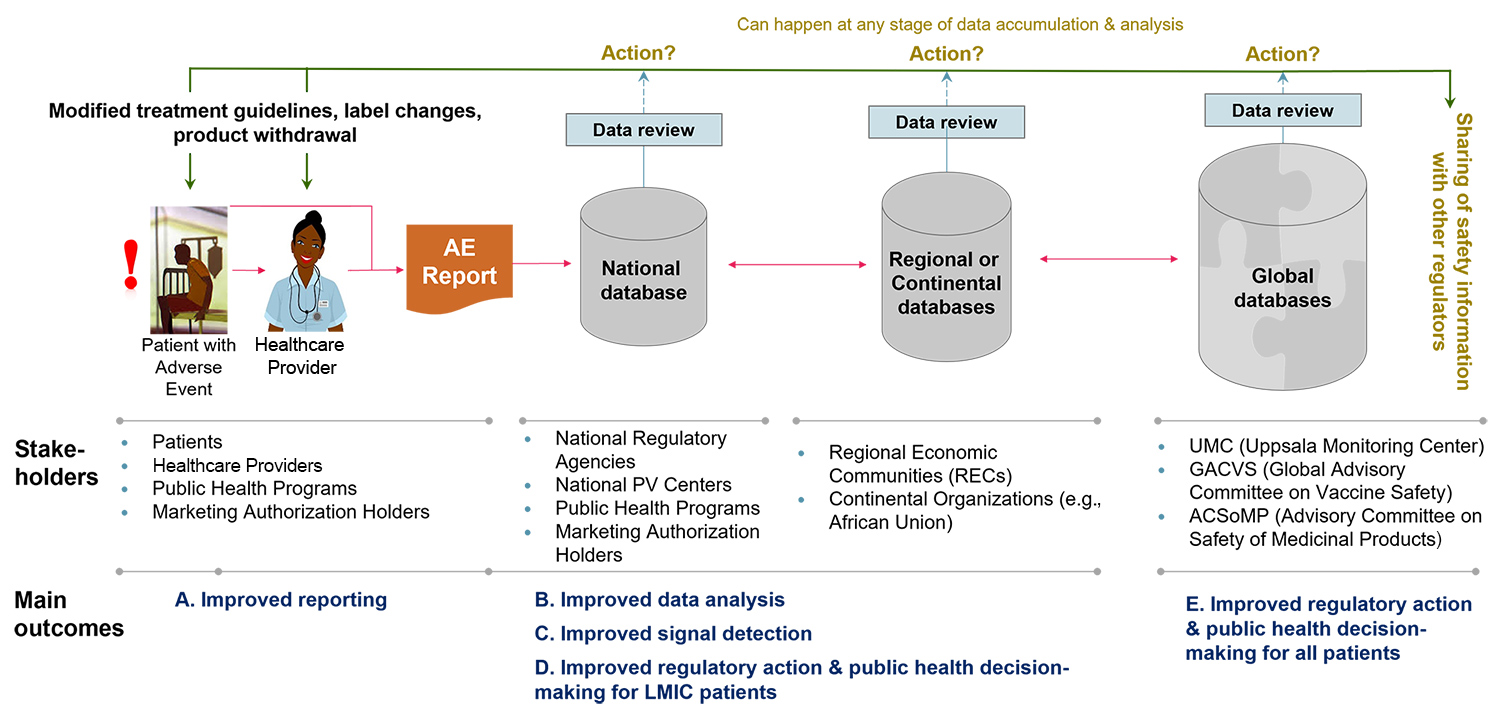
Figure 1 illustrates a functional PV system. Adverse events are captured in electronic formats and through interoperable systems become available in real time to PHPs and NRAs. These decision makers should have the ability to interrogate the data for causality and signal detection, as well as the expertise to make evidence-based decisions that link back to the patient and healthcare provider, such as changed treatment guidelines, labeling changes, or product withdrawal. Data should also flow transparently beyond the national level into regional and global databases and be continually analyzed for new signals that may become apparent as datasets are enhanced.
(This discussion focuses on in-country PV systems covering products once launched and not specific post-authorization studies required of product developers as part of their risk management plans.)
What is Smart Safety Surveillance (3S)?
The 3S approach is to use priority products relevant to the target country as the vehicle for PV systems strengthening. This allows often limited resources to be utilized in a focused way rather than attempting everything at once. By subsequently confirming or adding to the product’s safety profile, PHPs and NRAs can immediately show their public health impact to their patients and the value of the system strengthening to their stakeholders. PV systems can then be scaled in a sustainable way to other products and geographies of interest.
The first partnership in our 3S strategy was with the World Health Organization (WHO) and the UK Medicines and Healthcare Products Regulatory Agency (MHRA) to validate the initial 3S principles. Three new products (one vaccine, two medicines) with known safety concerns were chosen, as well as six target countries based on disease burden and some existing PV capability to build on: bedaquiline in Armenia, Brazil, Ethiopia, and Peru; rotavirus vaccine in India; and tafenoquine in Brazil, Ethiopia, Peru, and Thailand (due to tafenoquine launch delays, primaquine was used as a surrogate in some cases). Each country went through a baseline assessment using the PV module of WHO’s Global Benchmarking Tool (GBT) and then developed a customized strengthening plan for their specific gaps. Impact was measured based on reporting rates and level of structural indicators measured pre- and post-intervention, as well as whether the data analyses confirmed or added to the product’s known safety profile.
Within the nine- to 18-month pilot period (depending on the country), it was clear that the 3S approach sustained systems strengthening and positive public health impact (as detailed in a recent publication). Armenia, for example, took an end-to-end strengthening approach, including training for PHP and NRA professionals, rolling out the Med Safety app for electronic data collection, breaking siloes between PHPs and NRAs (bedaquiline safety data was not previously shared with the regulator), and creating a national pharmacovigilance committee to analyze and if needed act upon this data.
In contrast, India primarily focused on creating a process for integrating and analyzing data already being collected across multiple siloed safety programs (regulator, manufacturer, immunization program, sentinel sites, and academic studies), with an analysis of more than 1,500 cases of intussusception concluding that there was no added risk from the rotavirus vaccines used in the country’s immunization program. In both cases, the systems created were then scalable to other products.
Following this successful proof of concept, 3S has informed and become embedded in all BMGF PV strengthening partnerships in Central America, the Caribbean, Southeast Asia, China, and Africa. It was also chosen as the main theme of the 18th ICDRA (International Conference of Drug Regulatory Authorities).
Case Study: African-Union Smart Safety Surveillance (AU-3S)
The COVID-19 crisis provided an opportunity to accelerate this program and address an urgent new need: the imminent arrival of vaccines with limited to no safety data in African populations. In late 2020, AU-3S shifted its short-term strategy to help four pilot countries (Ethiopia, Ghana, Nigeria, and South Africa, initially targeted as COVAX entry countries and covering 30 percent of the African population) with their COVID-19 vaccine safety response. MHRA continues to be the main technical partner, with WHO AfRO (Africa Regional Office), WHO EMRO (Eastern Mediterranean Regional Office), and US FDA (US Food and Drug Administration) added as advisors.
Within nine months, working with each country’s NRAs and PHPs, AU-3S was able to roll out targeted in- and cross-country solutions that:
- Developed a digital health ecosystem for safety data collection (covering ~400M people)
- Strengthened safety capability end-to-end across both NRAs and PHPs
- Created an African-owned cross-country safety data integration and signal detection platform
- Created a cross-country Joint Signal Management group that reviews and acts on any signals seen in the integrated data.
The public health impact of the AU-3S COVID-19 vaccine pilot has been immediate and significant. Based on data collected so far (detailed further here, with more than 20,000 reports across four vaccines, and more than 1,600 vaccine-event combinations analyzed), African regulators and public health decision makers from the four pilot countries were able to confirm through their own analyses that these vaccines have similar safety profiles in their own populations as in HICs, and that the benefits of vaccination clearly outweigh the risks. As the continent receives more supplies (currently at less than 10 percent of estimated need) and the amount and diversity of available safety data continues to grow, the end-to-end system that has been created is ready and capable of responding to any potential new signals. AU-3S is now looking to scale this vaccine pilot to other countries, as well as potentially leverage the system for anticipated COVID-19 therapeutics. Moreover, other regions could use the approach taken by the African Union as a model to build their own accelerated pandemic safety response.
Moving Forward


