DT Consulting
n September 2021, DT Consulting, an Indegene company, launched the 2nd DT Digital Tracker, a methodology to assess the current state of digital technology adoption by clinical trial sites globally. The inaugural survey, which was published in February 2021 (see April 2021 Global Forum), showed that many clinical trial sites were still reluctant to integrate digital technologies into their processes and that cost, complexity, and finding the right technologies were the main barriers to digital adoption. The second wave of results shows that digital adoption is increasing and that concerns about patients’ ability to use digital tools is now the main barrier to digital adoption.
2021: Positive Year for Clinical Trial Research
Clinical research, medical technology (med-tech), and diagnostics continue to gain momentum. The previous report analyzed the number of clinical trials initiated each quarter starting in Q4 2019 and found a steady increase in clinical research. The latest analysis of the clinical trial database indicates that this momentum continues to increase. A significantly larger number of industry-funded clinical studies launched in the first half of 2021 compared with a year earlier (Figure 1). The number of studies that launched in Q2 2021 was 42% higher than in Q2 2020. A similar trend was seen for publicly funded studies: the number was 32% higher.
As faster FDA approval processes have also increased the drug approval rate, the focus on digital technologies adoption is now more important than ever to increase the chances of trial success, better data management, and more efficient drug development overall.
Emerging Trends in Clinical Trial Site Operations
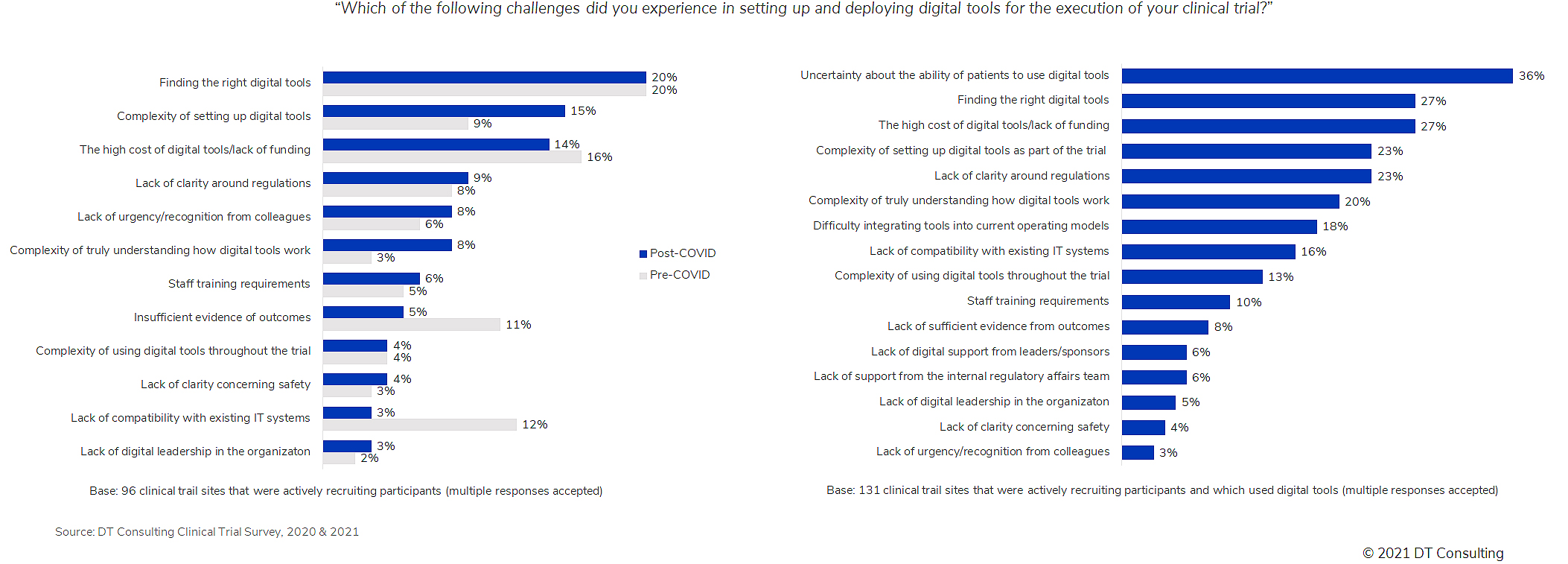
After March 2020, there was also a sharp increase in the percentage of respondents citing the complexity of technology setup (from 9% to 15%) and understanding how digital tools really work (from 3% to 8%) as challenges.
Conundrum of Digital Patient Recruiting and Unexploited Potential of RWE Approaches
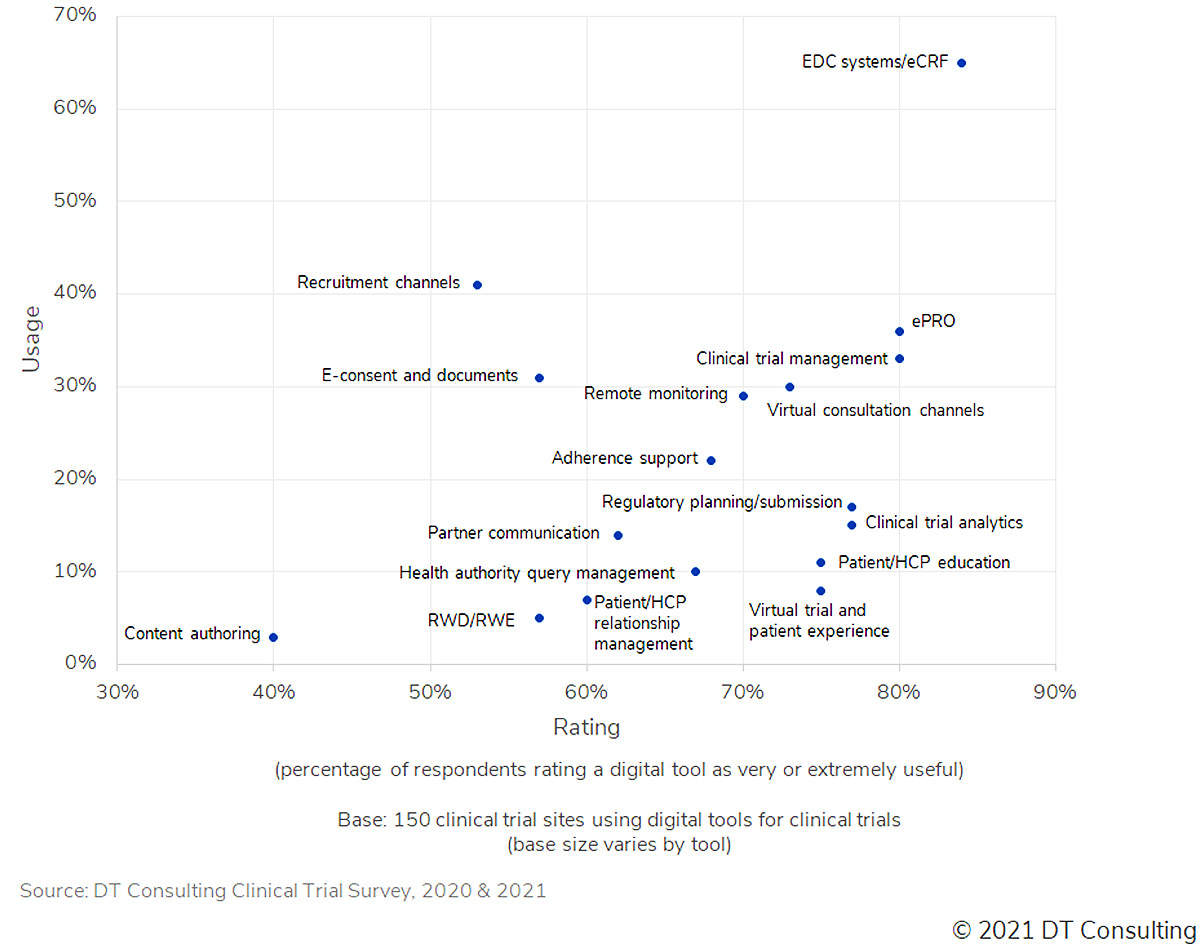
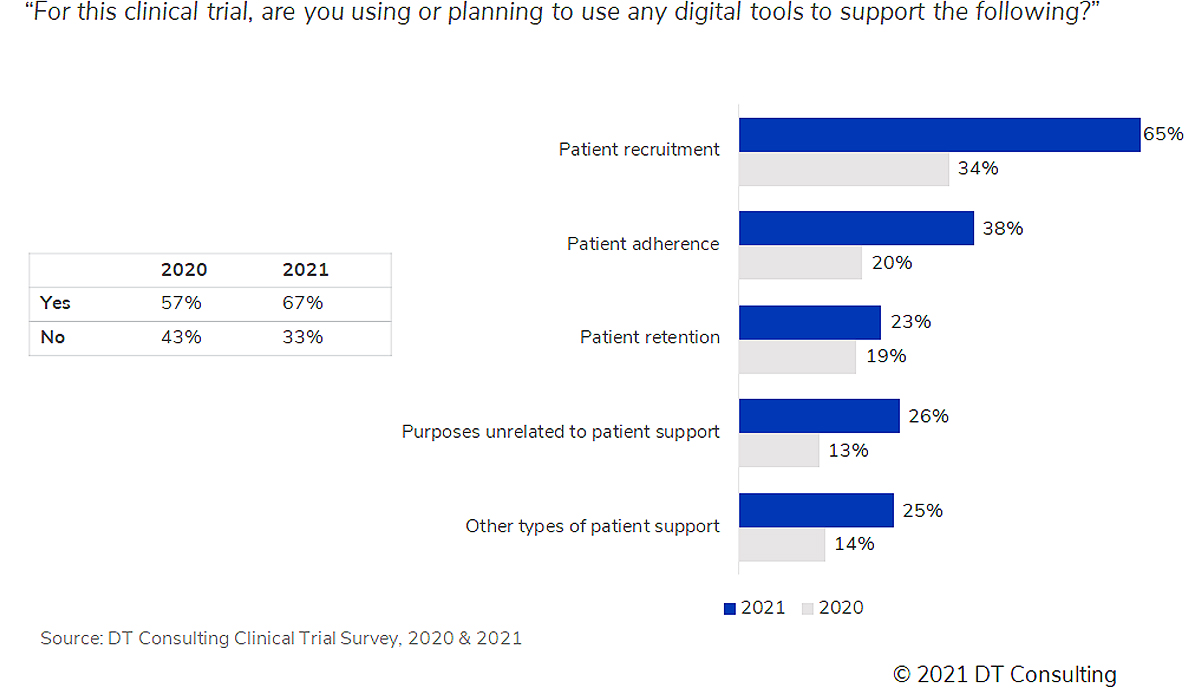
Overall, results show that the most used technologies are not necessarily the most effective. Sponsor support via clinical operations teams is critical to address challenges related to technology adoption, especially in assessing which new technologies could be more beneficial and facilitate data capture and flow. Our third DT Tracker Survey, which will be published February 2022, will specifically address sponsors-to-clinical sites interaction challenges.