Chair, DIA Board of Directors

Global Chief Executive, DIA
are experiencing the most substantial disruption in healthcare innovation in modern history. Emanating from the advent of systems biology, genetic and personalized medicine has led to a dizzying pace of change, resulting in advances in therapies and cures. And in today’s digitized world, the ability to capture, organize, cleanse, analyze, and act on the enormous amount of digital data is a challenge and huge opportunity for life science and healthcare professionals worldwide. At DIA, we have seen our stakeholder community collaborate to develop new levels of understanding, inviting new stakeholders from verticals that have not historically been players in our arena. We are in a new paradigm – and don’t expect things to slow down any time soon!
In 2019, we saw our digital transformation gain substantial momentum. Efforts initiated in years past led to several new knowledge resources, designed to ensure our community can gain personalized access to the latest thinking, engage with thought leaders, and share their own knowledge wherever and whenever they like, through our DIA NOW interface.
In our role as a Thought Catalyst, we also created and shared insights into transformative topics, such as the evolution of artificial intelligence (AI) and machine learning (ML) in healthcare; faster access to novel therapies, such as gene editing and microbiome and cellular therapies; perceptions of unmet medical need; real world evidence (RWE) in regulatory decision-making; and patient involvement in healthcare product development. Similarly, we broadened our global DIA Learning portfolio to deliver the expertise of subject-matter experts directly into the hands of healthcare professionals worldwide seeking to expand their skills.
What truly motivates us is to be at the table: asking the right questions in the right environments with the most expert voices involved, stimulating engagement and participation, and joining with all of you in the quest to deliver real value to our members.
We know that DIA is essential to our members, stakeholders, and the patients we ultimately serve. We look forward to continuing our work as a community of tens of thousands of life science professionals around the globe, and to collectively making a difference in the lives of patients.
Sincerely,



are a global association that mobilizes life science professionals from around the globe and across all areas of expertise to engage with patients, peers, and other experts in a neutral environment on the issues of today and the possibilities of tomorrow.
Engaging Patients
atient Engagement is one of the foundational areas in which we seek to develop thinking and generate insights that can be used to support decision-making for improvements throughout the medicines lifecycle that will result in better outcomes for patients. Through our thought catalyst, knowledge sharing, and interactive collaboration efforts, we strive to advance patient engagement in healthcare in ways that are beneficial to all stakeholders. Only then can advances last and become an integral part of stakeholder cultures.

Why We Care: We have long recognized the important role patients play in shaping the care they need. Increasingly, we recognize that the role ascends to the level of trusted partnership throughout the patient experience and the lifecycle of the medicine. Characterizing the ways that biopharmaceutical organizations successfully develop and sustain productive partnerships with patients and sharing that knowledge helps drive the development of healthcare products that better meet patient needs and deliver meaningful health outcomes.
- We released new findings of Phase II of the multi-phase study with the Tufts Center for the Study of Drug Development (CSDD) on patient-centric initiatives, Assessing Patient Engagement Preparedness, Capabilities, Experience, and Impact (PEPCEI). This aggregate report of industry experience with adopting patient-centric practices and sustaining patient centricity will serve as a benchmark for ongoing development in this area.
- Each of our regional and global annual meetings provided a platform and targeted sessions for patients and other stakeholders to share perspectives and new insights on meaningful partnership opportunities throughout drug development, approval, and access. In addition, the DIA 2019 Global Annual Meeting offered a dedicated track of patient-focused sessions providing cross-disciplinary sharing among these stakeholders.
- The DIA 2019 Global Annual Meeting and the DIA Europe 2019 Meeting provided full support for a combined total of 15 patient representatives to participate in these meetings and waived registrations for an additional total of 15. In addition to many opportunities to exchange knowledge and ideas, their participation resulted in four taped interviews, a podcast, and numerous articles in which patients shared their perspectives on collaborating in drug development. Several also joined the global DIA Patient Engagement Community.
- We worked with a team of patient engagement experts, including patient, non-profit, industry, and regulatory organization representatives, to develop a comprehensive competency framework of knowledge and skills needed to implement and sustain patient-centricity throughout the medicines lifecycle.
- Based on the patient engagement competency framework, we released a second eLearning module on patient engagement concepts for industry, entitled Preparing for Purposeful Patient Organization Partnerships. Including guiding principles on identifying appropriate partners and maximizing shared success, this module builds on the foundational concepts of patient engagement covered in the Introduction to Patient Engagement in Drug Development eLearning module.
- We strengthened patient representation in the governance of DIA by expanding the Patient Group Advisory Council (PGAC) formed in 2017, to the Patient Advisory Council (PAC), and elevating it to a strategic advisory body providing direction on the utilization of our global neutral platform to advance patient engagement in healthcare. As one of the four councils advising the DIA Board of Directors, the new PAC met for the first time in early November 2019.
- For the first time in Japan, the program of the DIA Japan Annual Meeting 2019 focused on patient and public involvement as a major topic of discussion. Attracting more than 1,200 participants, the meeting paved the way to further promote patient engagement in healthcare in this region.
- We participated in the International Liaison Group of the IMI PARADIGM (Patients Active in Research and Dialogues for an Improved Generation of Medicines) project (PILG) to advise and collaborate in the co-development of patient engagement practices through the project’s various work packages.
- We reported on additional insights from Phase I of our 2016 DIA-Tufts CSDD study on patient-centric initiatives in drug development in our article Assessing Biopharmaceutical Company Experience with Patient-centric Initiatives (PCIs) published in the peer-reviewed journal Clinical Therapeutics. The paper examined measures of impact that can be used to assess ROE for various PCIs and how resulting insights can be used to facilitate adoption of PCIs of highest impact for patients and biopharmaceutical research organizations.
n 2019, we continued to foster an environment for thought leaders to advance translational science by collaborating with industry, academics, patients, and regulators to turn new discoveries into tangible outcomes.
Why We Care: We recognizes the importance of translational science, which launches laboratory or clinical findings from mere observations to novel interventions that will improve patient outcomes.
- The 3rd DIA/FDA Oligonucleotide Therapeutics Conference featured an update on the antisense oligonucleotide therapy that was developed and administered for a single patient with Batten’s Disease. This project began during the first Oligo Conference, when Tim Yu, representatives from industry, and FDA came together to tackle the lack of available treatment for this disease and pioneer a new paradigm to develop a personalized treatment using an approved “N-of-1” clinical trial strategy.
- We completed the first phase of our Pharmatech project, which details the current uses and barriers to adoption for AI and other disruptive technologies in drug development. This project was done in collaboration with the Tufts Center for the Study of Drug Development. Results of this project were published in a peer-reviewed publication in the Journal of Clinical Therapeutics.
- Advanced Technologies and AI were also on the agenda at the 4th Cell and Gene Therapy Product Symposium in Japan. With the implementation of unique Japanese regulations in this area, brand new therapeutic regimens and issues concerning the collection of appropriate safety data and long-term follow-up were discussed by experts from Japan, US, and EU.
- Outcomes of the first DIA India New Paradigms in Translational Research and Early Phase Development conference include new collaborations with the South East Asia chapter of the American College of Clinical Pharmacology and Indian Council of Medical to work on translational research initiatives in 2020.
- Groups of key stakeholders discussed the challenges and opportunities in emerging areas of drug development at the DIA 2019 Global Annual Meeting, including the manufacturing of genome editing therapies and the use of real world evidence in regulatory decision-making.
s in previous years, our activities this year supported the global evolution of regulatory science at the intersection of cutting-edge scientific advances, current international regulation, and safe treatment access for patients. 2019 was an important year for several advanced therapies that provide patients with better quality of life and longer lifespans. With our ability to foster conversations among all stakeholders, we have made significant progress particularly in the areas of patient focused drug development and advanced therapies.

- Since we started discussions more than 15 years ago, our efforts to bring patients front and center in drug development culminated this year in the release of the FDA Patient Focused Drug Development Guidances. Our focus in this area has also turned increasingly global, with important advances especially in Asia: For the first time ever, the DIA Japan Annual Meeting 2019 and DIA China 2019 made patient focused drug development a leading topic to incentivize drug companies and regulatory authorities to address issues that matter to patients and their caregivers.
- In the gene-editing space, we fostered critical conversations among stakeholders at a various DIA meetings, bringing researchers and leading gene editing companies together with regulators to discuss manufacturing processes and create efficient regulatory pathways for cutting-edge gene therapies.
- This year, oligonucleotide-based therapies have finally come to market, and the first treatment for ALS is currently in development. With our steadily growing annual DIA/FDA Oligonucleotide-Based Therapeutics Conference we planted the seeds for important progress in this area by bringing together leading experts from industry and regulatory agencies to tackle the latest advances and challenges in the development of oligonucleotide therapeutics at all stages of the development process.
- We continued to explore applications of real word data (RWD) and real world evidence (RWE) to advance regulatory decision-making. Together with regulatory agencies from around the world we developed a landscape paper on currently available guidances and data. The article, Real World Data and Real World Evidence in Regulatory Decision Making: A Global Landscape of Guidances and Validated Data Sources, is in review and identifies challenges and ways forward for the use of RWD and RWE.
- As the only independent organization that brings together regulatory authorities from across the globe in an international Council of Regulators, we continued to advocate for and advance regulatory harmonization and technical standards through forums and trainings, including courses approved by the ICH (International Council for Harmonization). Notably, we hosted our first ICH-approved training workshops in India and Korea: the DIA India ICH Day 2019: Enhancing Clinical Trial Outcomes with ICH and the DIA NIFDS ICH PV Workshop: Best Practice in Pharmacovigilance with ICH E2 and RWD.
By fostering critical discussions among regulators, industry, and patient advocates at our Global Annual Meetings, we have ensured that the patient voice is integrated not only at the end stage of drug development but throughout the entire drug development process.

The DIA-CoRE Singapore Annual Meeting 2019. Speakers and members of the Program Committee line up for a group photo after a successful day dedicated to regulatory issues in the ASEAN region.
n 2019, our activities continued to build on the success of 2018 in three key areas to advance thought leadership in the value and access space: (1) sustainability of healthcare funding, (2) defining the concept of unmet medical need, and (3) evidence generation in the real world.
Why We Care: The year 2019 was marked with the first therapies that are going to change the payment paradigm coming to market. The first cures to genetic diseases, which came with high price tags and high uncertainty, triggered a wave of analysis of the suitability of the current system and need for new evidence, new payment models, and more discussion. It is therefore important to support the value and access community in raising difficult and exceedingly important topics for discussion in a safe, non-political environment.
- In collaboration with the Boston Consulting Group (BCG) Market Access Roundtable, the Sustainability of Healthcare Funding initiative progressed further, studying the emerging payment models—ranging from annuity payments, as promoted by companies such as Novartis, to subscription models—set forward by several US States. The three work streams finalized the data collection through workshop discussion (payment models), Delphi process (patient and cross-stakeholder engagement), and semi-structured interviews (data and infrastructure). The results are expected in early 2020 with dissemination at DIA Europe 2020, the DIA 2020 Global Annual Meeting as well as other select meetings. The payment models will be accompanied by better data usage and predictive technologies in waste reduction as well as better target identification.
- Our research on unmet medical need was published in the peer-reviewed journal Value in Health and presented at the EMA-EUnetHTA 2019 Meeting for senior officials from different institutions. In anticipation for the Commission report on orphan and pediatric medicines, which is expected to be released in early 2020, unmet medical need continues to be a high priority on our agenda.
- HTA and payer assessments were discussed in the DIA Future of Evidence Workshop in Basel that was built on findings from the previous DIA Value and Access Strategy Workshop, bridging the audiences from regulatory, access, and clinical.
What’s Next?
The new political cycle in Europe and the election campaigns in the US will keep the value and access topics high on the media and political agenda. Likewise, it will be among our top priorities to continue to provide a non-political environment to discuss and attempt to resolve some of these big challenges that relate to the sustainability of the sector, supporting our shared mission to make patients better.
o advance DIA’s mission, DIA Learning prepares life science professionals with the knowledge they need to develop timely medical products in today’s complex healthcare environment. The curriculum includes learning solutions that address training needs in safety and pharmacovigilance, regulatory affairs, clinical research, medical affairs, project management, patient engagement, and more.
Why We Care: At every stage of their career, life science professionals must stay abreast of crucial issues spanning the product lifecycle. It is critical to offer professional training that is current, effective, relevant, and trusted.

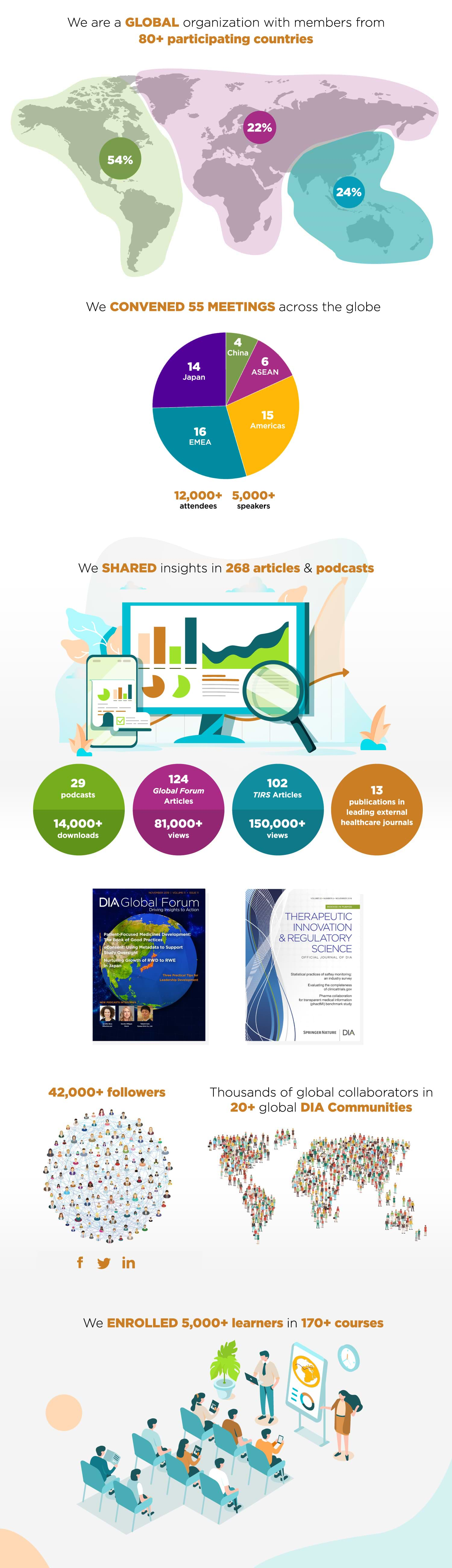
- In 2019, we held 140 training events around the world and more than 30 online courses to learners globally. More than 5,000 learners were trained worldwide. Especially in China, the number of learners has been on a steady rise.
- Eight hundred participants enrolled in the new Safety and Pharmacovigilance Certificate Program. This comprehensive program and defined Learning Path were developed based on the DIA Safety and Pharmacovigilance Competency Framework. Developed with experts working in the field, the Framework outlines the knowledge and skills needed to work in safety and pharmacovigilance.
- Learners enrolled in the Safety and Pharmacovigilance Certificate Program were issued 2,600 digital credentials. A digital credential is an online representation of an individual’s learning outcome, experience, or competency achievements. They can be shared and verified online.
New Online Courses
- There has never been more interest in fostering fruitful partnerships between life science companies and patient organizations. As with any new endeavor, before you get started it’s prudent to gain an understanding of the terrain of patient advocacy communities, changing capacities and expectations, and emerging best practices. The new Preparing for Purposeful Patient Organization Partnerships eLearning module provides an overview of different organizational types, how to identify and gauge potential partners, when to engage, what might be gained from partnerships, and guiding principles to maximize shared success.
- The updated Medical Communications eLearning Program eight-module program covers the key medical communication topics, including literature searching and evaluation, handling medical inquiries, writing medical responses, compliance, understanding study designs and statistics, product labeling, and crisis management.
- The new Basic Safety Sciences: Pharmacoepidemiology, Clinical Pharmacology, Pharmacogenomics, and Toxicology Studies eLearning module explains the principles of safety sciences to enable participants to analyze potential safety issues and to better understand the patient population and its experience with the drug. The module provides a broad appreciation for the activities and associated competencies needed to advance a drug candidate from an idea in a lab to a treatment ready to be studied in humans.
- The updated Clinical Trial Fundamentals eLearning Program includes three modules designed to provide a practical context to help clinical research professionals learn about conducting clinical trials.
- The updated Informed Consent: Comprehensive Concepts and Components eLearning Module explains the components of a complete and appropriate consent form as well as guidance for the creation and appropriate wording of these components.
cross corporate, regulatory, and international boundaries, DIA Communities and Working Groups serve as easily accessible hubs for stimulating peer-to-peer discussions, professional networking opportunities, lively idea exchanges, and high-quality content created by our members. Younger professionals and students can advance their careers and develop new skills by joining our new “Leader of Tomorrow” program and competing in the "Leader of Tomorrow" challenge.
Why This Matters: DIA Communities and our Leader of Tomorrow program offer DIA members at different career levels exclusive access to global conversations around healthcare. Community members work together to speed innovation in healthcare product development, producing resources such as tools, case studies, and best practices that benefit the entire healthcare ecosystem.
- In the second half of 2019, we relaunched the DIA Communities with an emphasis on ten key topics and reformulated our approach to member-driven content. This new approach aims to increase the number of programs and initiatives planned and led by members, with the DIA Communities as the central hub of their volunteer work.

- Activity in the DIA Communities continues to increase, with more than 14,000 logins and nearly 1,300 messages posted on the site in 2019. This increase has been driven primarily by activity within topic sections as community leaders use the platform to organize and advertise opportunities for members.
- Communities in China have also been very active and were behind five major events at the DIA China 2019 Annual Meeting. There are currently six active Communities in China.
- More than 3,000 searches were conducted within the DIA Communities in 2019, with GDPR and Regulatory Intelligence as the most popular search terms of the year.
- At the end of 2019, the Statistics and Women in the Life Sciences communities changed their names to Statistics and Data Science and Diversity and Inclusion in the Life Sciences, respectively. These changes were made to better reflect the missions of both communities to bring together members with a wide variety of experience and expertise.

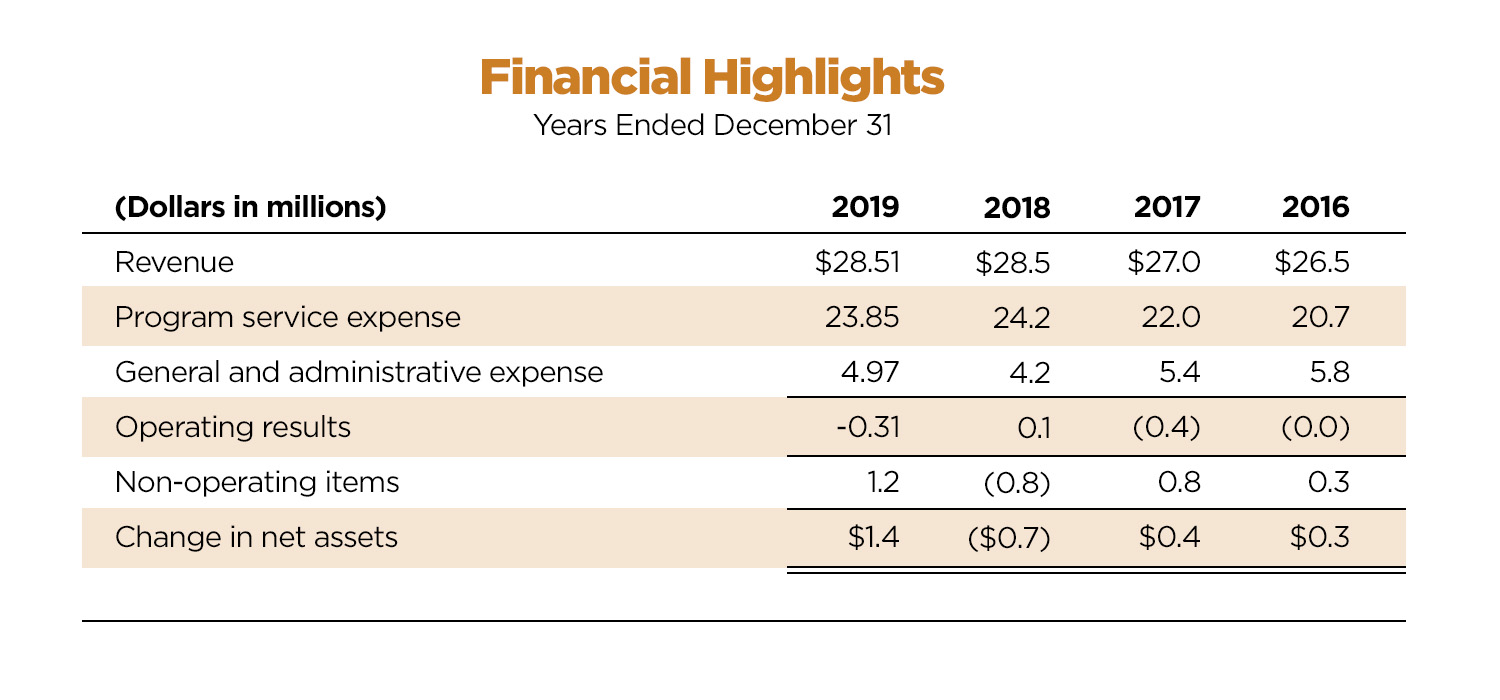
DIA Financials
or the year ending December 2019, DIA delivered a contribution to operating financial results, as well as a positive change in net assets.