Around the Globe
Get Ready to Adapt!
Roder
@Parexel
he European Union Clinical Trial Regulation 536/2014 (EU CTR) aims to standardize and harmonize the conduct and management of interventional clinical trials across the European Economic Area (EEA), with legally binding rules on requirements and increased transparency.
- Submit, evaluate (scientific and ethical review), and authorize clinical trial applications (CTAs);
- Submit any trial-related notifications, reports, and results, up to the clinical study report; and
- Serve as the single communication channel between the sponsor and the Member States Concerned (MSC) in the clinical trial.
The new EU-CTR promises a harmonized, simplified process designed to decrease the burden resulting from idiosyncratic interpretations of the current EU-CTD. But it also poses new operational challenges to many clinical trial sponsors.
The EU-CTR came into effect on January 31, 2022. From this date until January 30, 2023, sponsors can choose to submit CTA requests for new trials under the current EU Clinical Trials Directive 2001/20/EC (EU-CTD) or under the EU-CTR; however, after January 31, 2023, all new CTAs must follow EU-CTR processes. Sponsors must transition ongoing trials to EU-CTR by January 30, 2025.
Preparing for the EU-CTR requires wide-ranging cross-company initiatives. Companies that have started their preparations early understand very well that they must mobilize all stakeholders to analyze current business processes, upgrade information technology systems, and restructure operations to avoid disruptions to start-up, conduct, and maintenance of ongoing and new trials.
To Reap Efficiencies, Sponsors Must Sow Operational Changes
-
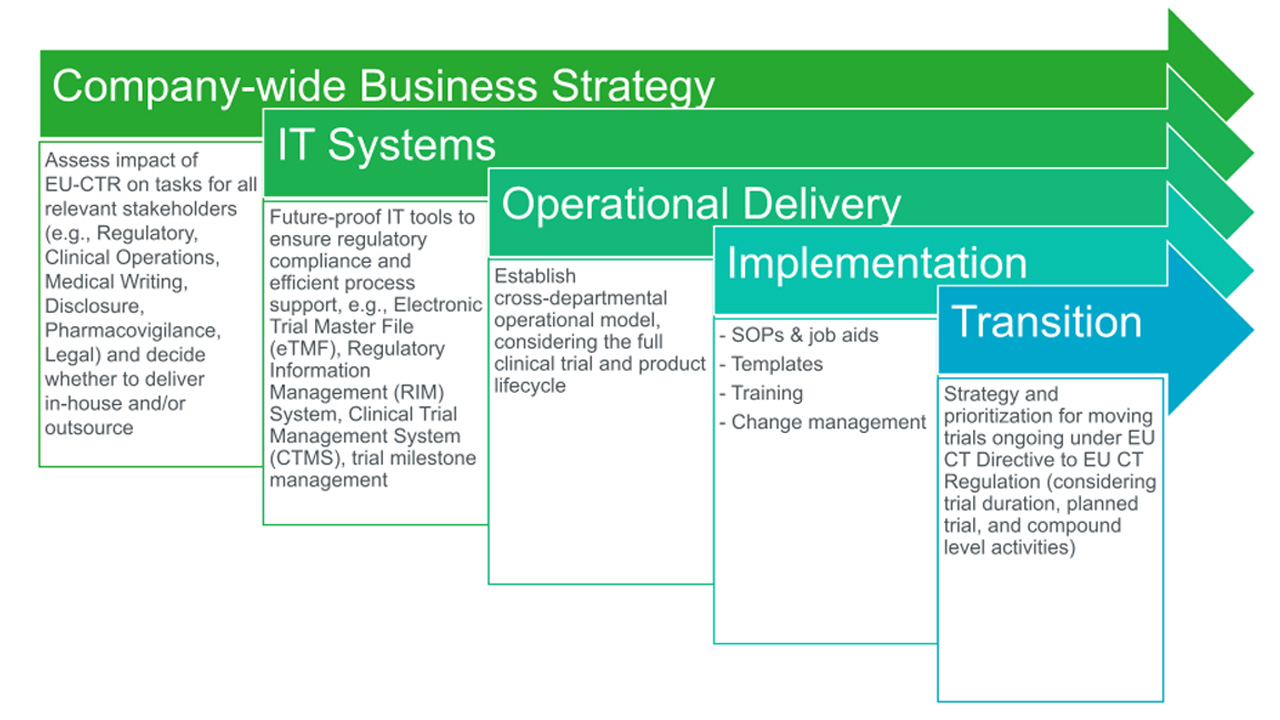
Analyze processes across your portfolio: To maximize efficiency, sponsors need to assess processes across their portfolio lifecycle. Early on, sponsors should engage with internal stakeholders and external outsourcing partners to communicate clearly defined processes and workflows that ensure business continuity and full compliance under the EU-CTR. They may need to employ different resourcing models to address varying circumstances. Sponsors should prepare for EU-CTR using a cross-departmental approach (Figure 1).
-
Focus on first-time quality: Under the EU-CTR, the success of a CTA depends on the first-time quality of the dossier to a greater extent than ever before. A CTA will not pass the validation phase in any MSC unless it receives clearance to proceed in all MSCs. You must complete the authorization procedure for an initial application before you can submit an additional MSC. This greater interdependency amongst MSCs in the trial demands that sponsors get applications right the first time.
-
Choose countries wisely: Under the EU-CTR, the study start-up strategy will need to change from “first EEA country ready” to a holistic approach to EEA country readiness. Part I of an EU-CTR CTA dossier evaluation is a joint assessment from all MSCs led by the Reporting Member States (RMS), proposed by the sponsors. However, if a CTA contains elements or provisions at odds with the RMS’s national laws, a negative Part I conclusion will affect all MSCs. Experts with local knowledge can tailor the country list. Choosing the right RMS and MSC will be critical.
-
Centralize CTA management: An industry-wide EU-CTR trend is centralization of CTIS management. Communication exclusively via CTIS and the short RFI response timelines call for a focused team that monitors the CTIS for incoming communications, handles document/data entry and download for trial master file compliance, and closely monitors timelines.
-
Upgrade IT infrastructure: Most companies will need to upgrade their electronic trial master file, regulatory information management, and clinical trial management systems to meet the new demands of EU-CTR. However, companies should realize that procuring and upgrading such systems can take anywhere from six to 12 months, and you can only upgrade effectively after a comprehensive impact assessment. It is vital to get the right insight and information about your current IT system and what you will need going forward.
-
Tighten timing: Under the EU-CTR, sponsors will need to coordinate initial applications—and all modifications—closely. Missed timelines may result in legal consequences, such as lapsed applications, for sponsors. Sponsors will need a strategic plan for management and oversight of timing the permitted and nonpermitted overlapping initial, substantial modification, and additional MSC applications.
EU-CTR will require tight process coordination by sponsor study teams. All contributors must synchronize activities—lifecycle planning for each trial as well as across all studies with the same product—because an ongoing assessment in one MSC will block further substantial modifications to Part I, or Parts I and II, for all MSCs. Sponsors should attempt to minimize in-process changes to a clinical trial and ask: Will this action impact the submission of another action?
If sponsors prepare well in advance, they will find that initiating and conducting clinical trials in a timely fashion will be manageable under the EU-CTR.
New Challenges to Many Clinical Trial Sponsors
Many small biotech companies may benefit from getting support from an experienced adviser to ensure they get ready for their first trial under EU-CTR. These EU-CTR experts can support sponsors by proposing best practices for EU-CTR readiness and providing guidance for trial submission content and timeline planning as well as advice on MSC selection. This is an important component to protect forecasted development timelines.
Not Yet Business as Usual: Plan Transitions Well in Advance
EU Clinical Trials Directive 2001/20/EC
EU Clinical Trials Regulation 536/2014
- Part I (core scientific data dossier): Assessment report by RMS (with contribution by each MSC)
- Part II (country-specific documents*): Assessment report by each MSC separately
*Informed consent documents, site suitability, etc.
EEA Countries: Austria, Belgium, Bulgaria, Croatia, Republic of Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, and Sweden.
EU CLINICAL TRIALS DIRECTIVE 2001/20/EC
EU CLINICAL TRIALS REGULATION 536/2014
- Part I (core scientific data dossier): Assessment report by RMS (with contribution by each MSC)
- Part II (country-specific documents*): Assessment report by each MSC separately
*Informed consent documents, site suitability, etc.
EEA Countries: Austria, Belgium, Bulgaria, Croatia, Republic of Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, and Sweden.

