eLabeling for Timely and Personalized Drug Information Updates to Physicians and Patients
Indegene
@indegeneinsight
he package insert (or prescribing information) is the pharmaceutical company’s key mechanism to communicate information about the drug to healthcare professionals (HCP) and patients, in line with national requirements. The approved labels are printed and distributed to the end users along with the medicinal products. Update and distribution of revised prescribing information is often delayed due to the physical nature of the label, which can lead to a potential risk to patient safety.
Shortcomings of Physical Package Insert/Label
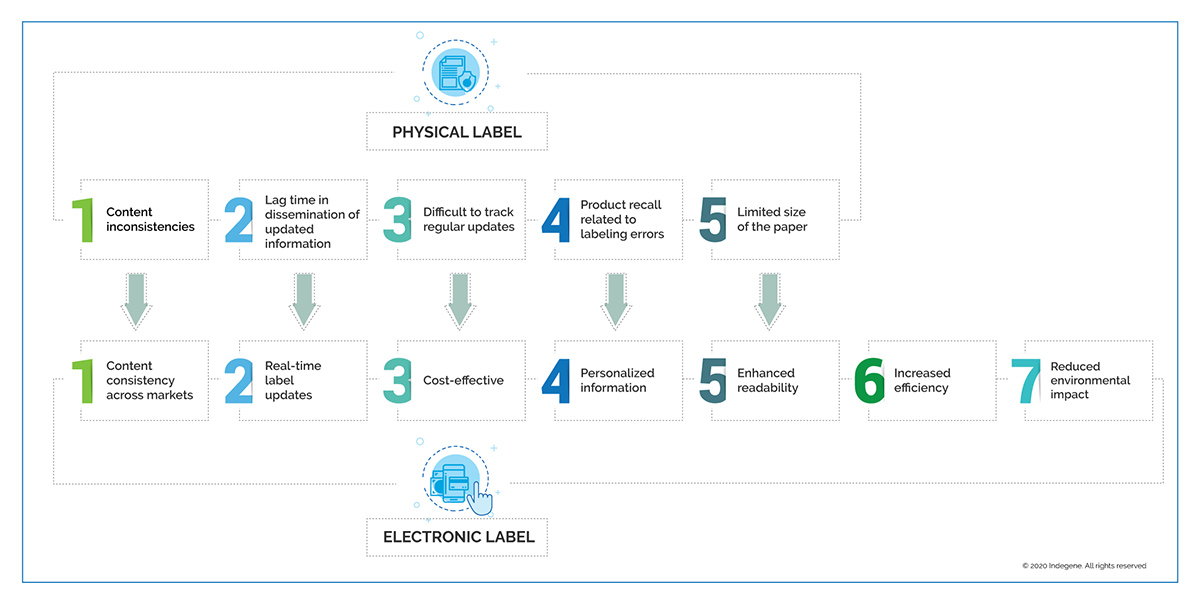
The challenges presented by a physical label are due to its inflexible nature and non-scalable processes. Other challenges include content inconsistencies across markets due to region-specific regulations, and increased cost associated with physical labels. Often due to limited paper size, text is cramped on the label by using small fonts, which impairs readability. In addition, physical labels provide no personalized information and the patient must search for relevant text. Pharmaceutical companies encounter multiple problems in tracking and implementing frequent label updates in order to provide accurate information to patients and HCPs, and the physical nature of labels delays dissemination of safety information.
Change Underway
Benefits and Opportunities
The PIL contains regulator-approved information pertaining to all registered medical conditions specific to every age group using the drug. It also contains an exhaustive list of adverse events for these conditions. Such detailed information can overwhelm patients and, in some cases, cause them to miss or overlook important dosing instructions.
Employing artificial intelligence (AI)-based solutions and content management systems can reduce cycle times and deliver faster label updates. An eLabel can:
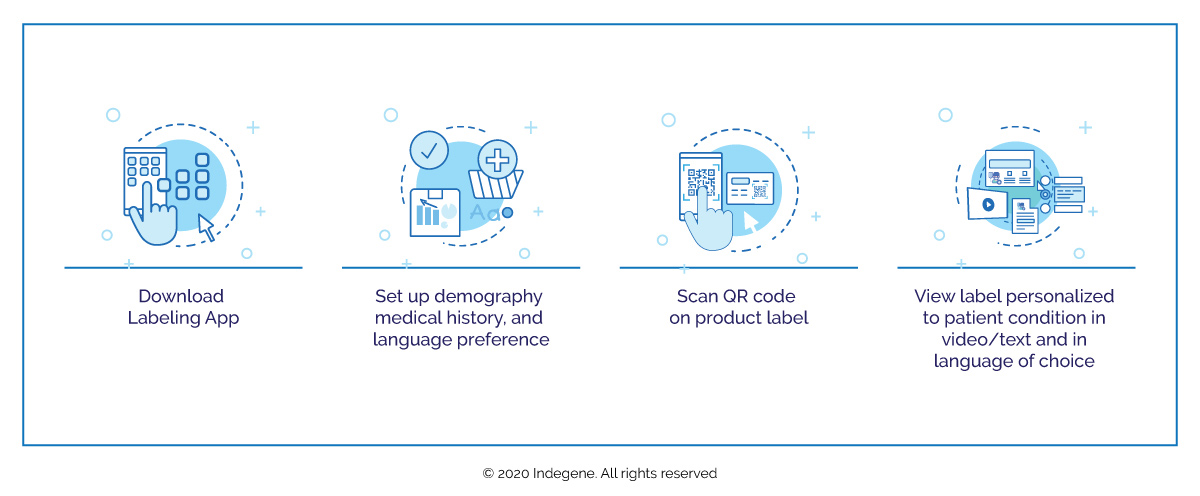
- Deliver personalized, user-friendly information directly relating to the patient’s medical conditions in video/text and in the language of the patient’s choice
- Accommodate a search function, facilitating easier access to information
- Enable individuals with impaired vision to change the label font size
- Enable audio readouts
Such adaptation can produce consistent quality documents with reduced manual intervention. Automated processes can also improve compliance, efficiency, and (above all) patient safety.
Challenges in eLabeling Landscape
Evolution in the global pharmaceutical market has made the shift to eLabeling inevitable and industry is moving towards this new form of delivering prescriber information. Various regulatory agencies are working towards refining guidance for implementation of eLabeling but the need for harmonization remains.
In 2015, FDA mandated electronic distribution of the package insert for companies marketing their products in the US. Currently, FDA-approved labeling documents are made available on its official website, DailyMed. Not much progress has been reported to interlink eLabeling after this guidance was released, leaving a gap that must be filled.
FDA’s 2015 eLabeling guidelines faced tough resistance from multiple quarters right from the start. The Institute for Safe Medication Practices (ISMP) commented that “to find right information, users have to sift through numerous pages to locate the actual drug information that they are seeking." FDA also received comments that eLabeling might impact how physicians treat their patients, which could lead to workflow disruptions, and that eLabels would not be readily available to healthcare professionals when prescribing decisions must be made.
To make eLabeling a success, planning and harmonizing labelling information to comply with local regulations, along with effective collaboration between regulators and manufacturers to design a robust process, is necessary. One of the foremost obstacles in implementing eLabeling is the lack of universal regulations or rules. A subset of the patient population is not currently equipped with mobile phones or are not tech savvy. Present regulations do not mandate eLabels, which remain optional, while paper labels continue to be mandatory. Both physical and eLabels will co-exist in the interim, and regulators and pharmaceutical companies will have to focus on both to dispense updated information to end users in real time.
In addition, pharmaceutical companies implementing eLabeling would incur one-time costs to acquire and build supplementary hardware infrastructure and to train stakeholders. Regulatory reluctance to adopt new technologies, lack of proper equipment and technology support, and lack of ownership for hosting databases present other pertinent challenges.
ASEAN, Canada, Europe, and Japan
In 2012, the EMA released detailed guidance on format requirements for electronic submission of product information. Another report from the European Commission (EC) was published in March 2017 to improve and enhance readability, and to guide developing an electronic format that meets patient needs. In 2018, EMA and EC organized a workshop to create alignment across various stakeholders on common, key European Union principles and pave the way for implementing electronic product information in the EU. In January 2020, EMA issued guidelines to implement electronic product information (ePI) and allow its dissemination via the world wide web and other ePlatforms.
Japan is leading eLabeling implementation and issued revised implementation process guidelines in 2019. The Japan Pharmaceutical Manufacturers Association requires product information in SGML and HTML formats and has also proposed guidelines for electronic labeling.
Health Canada is also accepting drug information in XML format on a “by request” basis, which most observers anticipate will eventually become mandatory.
The regulatory environment for eLabeling is still evolving in the ASEAN, and nations such as Australia, Singapore, and Malaysia have adopted and defined standards for structured labelling text. Health authorities (HA) in Taiwan have published a phone app which can scan 2D barcodes from drug cartons to access information directly from the HA website.
Making eLabels a Reality
Robust content management powered by AI, along with integrated content models to ensure consistent content across different markets, are required to make eLabels a reality.
AI combined with Natural Language Processing (NLP) business rules can be overlaid on specific country requirements to manage drug label processes with minimal human intervention. In addition, label personalization requires strong data analytics and robust business rules for accessing information from the HA website. Content mapping will automate the flow of information from multiple data sources to the appropriate product label.
Another trending technology initiative is an intelligent content-enabled labeling solution integrated end-to-end to manage labeling content. This platform uses AI and machine learning (ML) technologies such as natural language processing or understanding (NLP/NLU) to effectively convert labeling documents into a digital format based on semantics, which will significantly reduce lead time while ensuring compliance and consistency. The platform can deliver information in XML, JSON, and HTML formats. This not only ensures wider and more effective delivery of labeling content, but also ensures that updates to labeling documents can be made faster and more accurately and that information is disseminated to HCPs and patients effectively and efficiently using eMails, websites, NLMs, and mobile apps.
Conclusion
The non-profit collaborative TransCelerate BioPharma Inc. is working with pharmaceutical companies globally to create solutions and address barriers that inhibit efficient patient care. They are also trying to address issues by creating awareness and facilitating eLabel initiatives with a patient-centric approach. This includes a “tool kit” that provides an overview of activities helpful in implementing eLabeling and can gather stakeholder feedback. TransCelerate is also trying to collaborate with health authorities to evolve regulatory requirements.
Each market’s attitude differs due to the local country regulations. Adopting eLabeling would require amending local regulations, which places additional responsibilities on regulators and drug manufacturers.
We clearly see a greater momentum in the implementation of eLabeling as we look at progress made across the globe. There is a compelling need to automate labeling processes to ensure content consistency across markets, personalized product information, and enhanced traceability, thereby improving patient safety and brand integrity.