From RBM to RBx:
The Keys to Successful Adoption and Implementation
CluePoints
The Foundation of RBx
he latest version of the Good Clinical Practice (GCP) quality standard insists that sponsors implement a risk-based approach to study execution, a methodology that is now widely referred to as “RBx.” This goes far beyond the previous guidance from ICH, FDA, and EMA, advocating the adoption of risk-based monitoring, to now addressing all areas of quality management, not just monitoring. The areas of the guidance which address the new risk-based quality management and CRO oversight requirements can be found in the following sections of ICH E6 R2:
- Section 5.0: Quality Management (Risk-Based Quality Management)
- Section 5.2: CRO (Oversight)
- Section 5.18: Monitoring (Risk-Based Monitoring)
Executing clinical trials in line with these recommendations can be challenging for sponsors and CROs. This article will highlight how a risk-based approach to study execution (RBx) can help support traditional monitoring, medical monitoring, and data management by utilizing statistical algorithms to determine the quality, accuracy, and integrity of clinical trial data, both during and after study conduct and, in so doing, facilitate compliance with regulatory requirements.
ICH E6 R2 and Quality
The motivation for this significant paradigm-shift in quality management is explained directly in the introduction section of the ICH E6 guideline. It points to a couple of key factors that have emerged over the past 15 to 20 years. First is the rapidly increasing complexity and cost of clinical research. The second is the transition away from largely paper-based research to the modern approach of most studies adopting electronic and digital technologies such as EDC, ePRO, IRT, and others. This move away from paper has opened a tremendous opportunity to plan and manage clinical research more effectively and efficiently using RBx methodology, a very timely development to address the growing crisis in research complexity, duration, and cost. This increase in complexity poses ever greater challenges to achieving quality outcomes, as both patients and sites are burdened with managing a myriad of requirements placed in front of them.
Quality applies to every aspect of a clinical trial and it starts with the protocol and design of the study. Fundamentally, the approach to data quality should be efficient and avoid unnecessary complexity. Section 5:0 Quality Management states:
“The sponsor should implement a system to manage quality throughout all the stages of the trial process. Sponsors should focus on trial activities essential to ensuring human subject protection and the reliability of trial results. Quality management includes the design of efficient clinical trial protocols, tools, and procedures for data collection and processing, as well as the collection of information that is essential to decision-making.”
“The methods used to assure and control the quality of the trial should be proportionate to the risks inherent in the trial and the importance of the information collected. The sponsor should ensure that all aspects of the trial are operationally feasible and should avoid unnecessary complexity, procedures, and data collection. Protocols, case report forms, and other operational documents should be clear, concise, and consistent.”
Risk management is a process that underpins the overall quality of the trial. Identification, control, and communication of risk are important steps to ensure the overall quality of the study. Risk management encompasses study planning through to execution. Again, section 5.0 Quality Management states that:
“The quality management system should use a risk-based approach as described below:
5.0.1 Critical Process and Data Identification
5.0.2 Risk Identification
5.0.3 Risk Evaluation
5.0.4 Risk Control
5.0.5 Risk Communication
5.0.6 Risk Review
5.0.7 Risk Reporting”
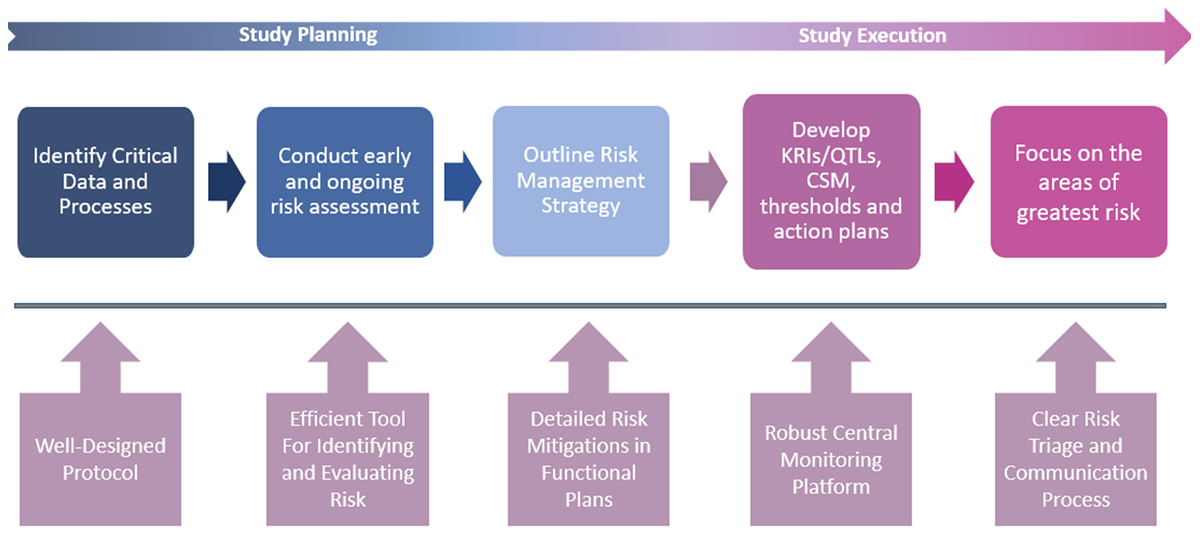
It is important to understand that Quality by Design (QbD) and Risk-Based Monitoring (RBM), two important concepts promoted in the ICH update, should not be addressed as separate ideas but as two phases of the same RBx paradigm. Both are focused on improving the operational success of clinical research, and both apply the core process of risk assessment and risk mitigation, plus the required documentation that goes with both. QbD becomes RBM once a study protocol is finalized, at which point risk assessment is repeated with the goal of mitigating any remaining operational risks. Mitigation plans are then applied during study execution, which includes ongoing risk monitoring and a more targeted approach to site monitoring. The following diagram presents this workflow.
Centralized Statistical Monitoring (CSM) is a critical component of the operational success of RBx, as it is a key and under-used weapon for quality oversight. An effective centralized monitoring approach should include the following three components:
- Data Surveillance
- Key Risk Indicators (KRIs)
- Quality Tolerance Limits (QTLs)
Quality is much more important than quantity, especially as it relates to KRIs and QTLs. It should not be necessary to implement 30 or 40 KRIs for each study, which will be challenging to maintain and inevitably lead to duplicate risk detection and greater risk of “signal noise,” causing study teams to waste time chasing down false risk signals. Rather, identify a core set of appropriate KRIs, and focus on ensuring that these KRIs are optimized for earliest possible detection of risk and for minimizing likelihood of false alerting. The same principle should apply to QTLs, which should focus on the most important study level risks or “failure points.”
Data Surveillance, also referred to as CSM, has been under-appreciated by many organizations in terms of the level of importance it has in effective quality oversight. While KRIs and QTLs are designed to monitor for pre-identified areas of risk, Data Surveillance can be effective at exposing various forms of study misconduct that may be more difficult to identify and/or characterize during pre-study risk planning. Data Surveillance works most effectively by running a discrete set of well-designed statistical tests across a broad swath of study data in order to identify atypical patterns of data at various sites that represent potential misconduct—whether intentional or unintentional. The forms of misconduct will range from outright fraud, to sloppiness and training issues, and will even relate to malfunctioning or incorrectly calibrated study equipment.
CSM is at the heart of RBx. CSM interrogates all clinical and key operational data to find anomalies and discrepancies that remain undetected using traditional techniques. CSM is more than just computing statistics on a subset of key variables. It is about processing all data and guiding users to where the potential issues in the data are. RBx relies on a combination of different tools. A central monitoring platform acts as the enabling technology, encompassing central data review, risk assessment (RACT), KRIs, data quality oversight, and issue and action tracking management systems. Risk findings should be documented thoroughly and accurately for FDA inspection purposes.
Controlling Risk – Proactive Data Monitoring
The first step in proactive data monitoring is to identify what is possible to mitigate, eliminate, and accept. This all forms part of various plans, including data plans, training plans, monitoring plans, statistical analysis plans, safety plans, medical monitoring plans, quality plans, and other functional plans. Key risk indicators, quality tolerance limits, and central statistical monitoring are all crucial to the process in order to identify risk signals and comply with the regulatory obligations.
Risk communication is crucial. The entire study team should be aware of the risks and how they are being managed. It is important to establish a triage process for communicating risks, follow up investigations, and corrective actions. The right person, the right information, at the right time is the best approach to decide how and when to act. It is crucial to provide a means to record follow-up, corrective actions, and outcome. A signal and action tracker, for instance, would be invaluable. Risk review and continuous improvement should be maintained throughout the trial, and risks should be re-evaluated throughout the study (e.g., enrollment versus treatment versus follow-up). Not every risk may be identified initially, which highlights the importance of “unsupervised” data review. Crucially, risk assessment and risk management should be part of EVERY trial.
RBx Implementation Success
An important first step towards ensuring sustainable success in adopting RBx is to establish and confirm the primary objectives for adopting the strategy. In other words: What is the organization trying to achieve with RBx? Each of the following three dimensions of value should be considered:
- Improved Quality – resulting in more reliable stage-gate decisions and faster time to market.
- Reduced Operational Costs – particularly resulting from a more adaptive, targeted approach to site monitoring. The savings achieved can be very significant per study as RBx becomes fully implemented.
- Shorter Timelines – specifically due to more effective enrolment and retention, along with a more efficient path to study database locks.
It will be additionally beneficial to identify and implement quantitative success measures to enable periodic assessment of the impact that RBx implementation is having toward the stated objectives.
Looking to the future, RBx can also be applied across studies, programs, and organizations alongside machine learning and artificial intelligence to identify program, organization, or industry trends. Learning from historical data can help make future decisions to support study setup, risk assessment, analytical tools, and signals management.
Conclusion
RBx implementation can be overwhelming for an organization given the wealth of information that is currently available. Starting simple is one way to maintain focus and concentrate on the elements of RBx that are most important to success. This principle applies not only to initial rollout, but also as an organization reaches steady state and RBx becomes “business as usual” for the organization.
Risk-Based Study Execution (RBx) – It’s Time to Close the Loop!
Those who have achieved a successful rollout of RBx and are approaching a steady state environment have enabled this success by applying thoughtful but simple processes, smart enabling technology, and a focus on change management. All the key components of RBx implementation (including pre-study risk planning, adaptive site monitoring with a significant reduction in Source Data Verification [SDV], and centralized monitoring) do not need to be overly complex to be effective. Elements of RBx can be implemented individually and independently with great success, making clinical trials better, faster, and cheaper for sponsors and CROs.

