Next Steps in Immunotherapy for Precision Oncology
Jianda Yuan
Eric Rubin
Emmett Schmidt
Alexandra Snyder
Merck & Co., Inc., USA
mmune-checkpoint blockade (ICB) therapies targeting cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death 1 protein (PD-1) or its ligand, PD-L1, have led to durable clinical efficacy in multiple cancer types. This has resulted in a paradigm shift from treatment approaches focused on tumor cytotoxicity to those that modulate the host immune response. While ICB monotherapies have demonstrated clinical benefit in a substantial proportion of patients, a majority still do not respond. Increased understanding of the resistance mechanisms to ICB therapies and biomarkers predictive of response is needed to address this unmet clinical need, expand the role of monotherapy, and guide the future development of effective combination therapies.
As such, we are at the end of the beginning of an era that promises even greater breakthroughs in cancer care. In this article, we describe two approaches towards achieving precision immunotherapy:
- Maximizing monotherapy potential using predictive biomarkers.
- Evaluating a diverse spectrum of combinations therapies.
Maximizing PD-1/L1 Antibody Monotherapy Potential
The immune checkpoint molecule PD-1, expressed on activated T-cells, is considered a marker of T-cell exhaustion. Treatment of patients with therapeutic antibodies targeting PD-1 (pembrolizumab, nivolumab, cemiplimab) and PD-L1 (atezolizumab, avelumab, durvalumab) has resulted in FDA approvals in a wide range of metastatic diseases. Clinical efficacy with anti-PD-1 monotherapy was first demonstrated in patients with advanced melanoma, and led to the first PD-1 ICB FDA approvals. Subsequently, agents that target PD-(L)1 have shown encouraging clinical benefit in more than 25 tumor types, including classically immunogenic tumors, such as melanoma and renal cell carcinoma (RCC), as well as challenging tumor types, such as non-small cell lung cancer (NSCLC), head and neck squamous cancer, gastric, cervical, and ovarian cancers, among others.
Although broad clinical efficacy has been demonstrated with PD-(L)1-targeting agents, responses are heterogeneous between and within tumor types. Maximizing the potential of PD-(L)1 monotherapy requires a better understanding of the mechanisms that limit cancer immunotherapy. In this regard, predictive biomarkers that select patients likely to derive the most benefit from ICB monotherapy are essential. Expression of the PD-L1 ligand in tumors has been validated as predictive biomarker for anti-PD-(L)1 therapeutics for several tumor types and is required as a companion diagnostic in some indications, including advanced lung cancer with or without prior treatment, and cervical and advanced/metastatic urothelial cancers that have progressed after chemotherapy. In other cases, PD-L1 testing is not required, particularly in combination settings, such as the combination of chemotherapy with anti-PD-(L)1 therapies in lung cancer.
In addition to PD-L1 IHC, the clinical utility of measuring tumor microsatellite instability (MSI-H) or deficiency in DNA mismatch-repair proteins (dMMR) has been validated by the FDA approval of pembrolizumab for the treatment of patients with MSI-H/dMMR tumors, regardless of tumor type. Similarly, nivolumab was recently approved for MSI-H/dMMR colorectal cancers. In addition, tumor mutational burden (TMB) is emerging as a potential predictive biomarker for ICB therapies.
Interestingly, TMB and inflammatory markers (PD-L1 or gene-expression profiling [GEP]) are independently predictive of response to pembrolizumab and atezolizumab in some settings. Patients with both high TMB and inflammatory biomarkers (PD-L1 expression or a GEP reflecting interferon-γ expression at the tumor) were shown to have higher clinical responses to pembrolizumab monotherapy across multiple tumor types. Evaluation of these biomarker-defined subgroups revealed potentially targetable resistance biology in a large genomic database.
Evaluating a Diverse Spectrum of Combination Therapies
Many patients do not respond to single agent PD-(L)1 due to primary and acquired resistance, the study of which is in early stages. Resistance may involve upstream deficits in antigenicity or antigen presentation as well as tumor-intrinsic and -extrinsic T-cell activities in the tumor microenvironment (TME; see figure). Some of these resistance mechanisms may be overcome by combining ICB therapies with other immunomodulatory agents, while others will require orthogonal interventions. The immunomodulatory effects of conventional and targeted systemic anti-cancer agents, as well as radiation and surgery, have been attributed to the replenishment of immune surveillance by enhancing antigen shedding or presentation and/or by overcoming adaptive immune-suppression in the TME (alterations in immune-regulatory receptors, ligands and cytokines, activation of innate-immune pathways, favorable effects on immune-regulatory cells).
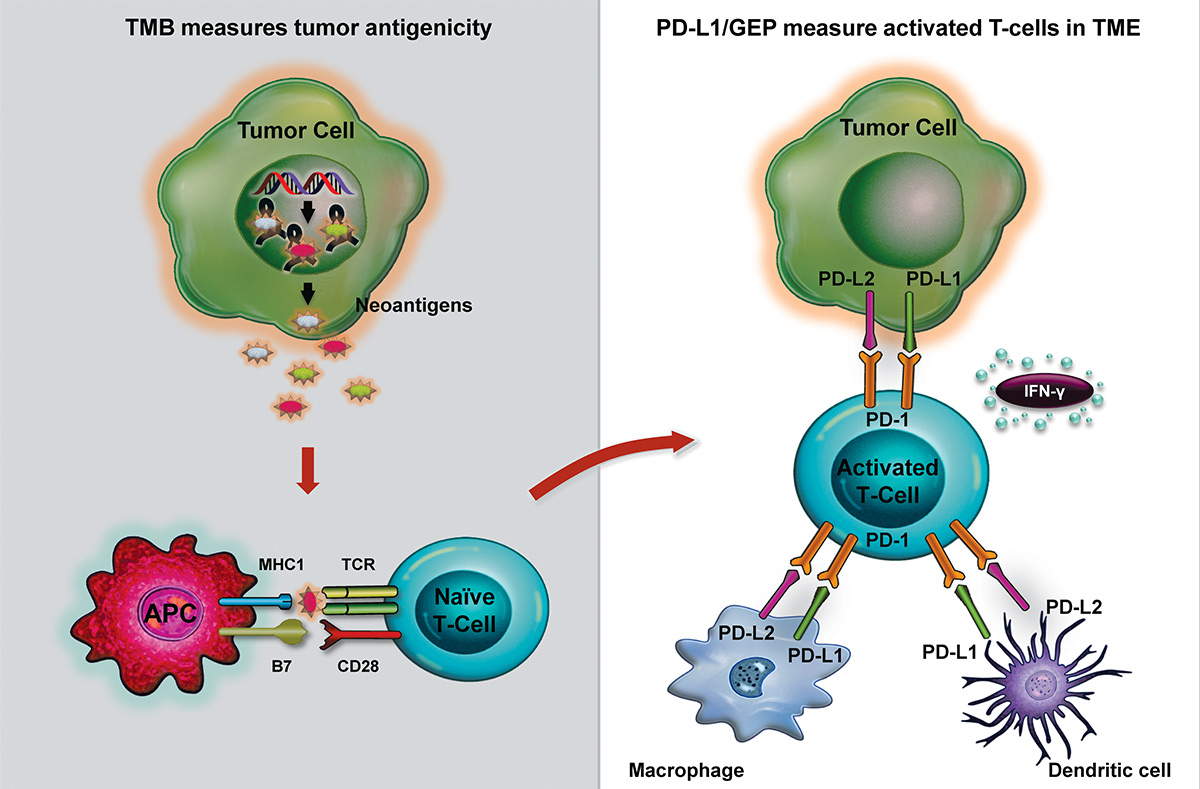
Tumor mutation burden (TMB) and PD-L1 immunohistochemistry (IHC) or gene expression profiling (GEP) assess different biology.
TMB is a surrogate biomarker of tumor antigenicity. Tumor somatic mutations may give rise to neoantigens distinct from self-antigens that, when presented to T-cells by major histocompatibility complexes (MHC) on antigen-presenting cells (APC), such as dendritic cells, can result in the activation and infiltration of T-cells into the tumor microenvironment (TME). The interaction of activated T-cells with tumor cells in the TME leads to the upregulation of PD-1 expression on T-cells, and PD-L1 and PD-L2 on tumor and immune (e.g., dendritic and macrophage) cells, as well as interferon (IFN)-γ-related gene expression. PD-L1 IHC stain and GEP are inflammatory biomarkers indicative of PD-L1 expression and activated IFN-γ-related genes, respectively, in a T-cell inflamed TME.
The need to increase the proportion of benefitting patients, combined with the current understanding of therapeutic mechanisms, has fostered the design of combination therapy strategies. These therapies can be broadly categorized into interventions that modify the behavior of T-cells and other immune populations in the TME, modulation of metabolism, induction of immunogenic cell death, or combinations of these effects. Combinations of ICB with chemotherapy have shown particular promise, presumably related to the immunomodulatory effects of chemotherapy. Nivolumab in combination with chemotherapy showed preliminary efficacy in NSCLC. In addition, pembrolizumab combined with chemotherapy demonstrated favorable clinical response in patients with NSCLC (KEYNOTE-021, G-cohort) leading to FDA approval. Recently, the phase III KEYNOTE-189 and -407 studies showed improved clinical response and superior overall survival with combination chemo-immunotherapy versus chemotherapy in non-squamous and squamous NSCLC, respectively. Furthermore, a study of chemotherapy and bevacizumab with or without atezolizumab in NSCLC showed a survival advantage for the quadruplet versus the bevacizumab-containing triplet. ICB in conjunction with systemic chemotherapy is also being tested in other malignancies.
Among checkpoint combinations, combined PD-1 and CTLA-4 blockade with nivolumab and ipilimumab, has shown promise in metastatic melanoma, renal cell carcinoma (RCC), small cell lung cancer and NSCLC, although a survival advantage has yet to be demonstrated relative to anti-PD-1 treatment alone. Many other combination immunomodulatory strategies are underway, including T-cell agonists and antagonists, cytokines, and intra-tumorally injected agents ranging from oncolytic viruses to innate immune activators.
The clinical utility of individual biomarkers in combination therapies may differ. PD-L1 and TMB have been evaluated as predictive biomarkers for combinations with atezolizumab, nivolumab, and pembrolizumab with varying success. A precision oncology clinical trial designed to evaluate specific pembrolizumab-based combination therapies in TMB- and GEP-defined patient groups in first-line NSCLC patients (KEYNOTE-495, KeyImPact study, NCT03516981) is underway.
There are myriad challenges in developing combination therapies, largely because of the nearly limitless permutations of combinations and a paucity of preclinical models to precisely guide the selection of therapies, doses, schedules, and disease indications. Furthermore, clinical data generated in phase I/II clinical trials do not consistently translate to a positive randomized phase III trial.
Future Perspectives
The impressive clinical results achieved with ICB therapies across multiple tumor types have dramatically changed the landscape of cancer treatment. However, additional therapeutic strategies are needed to overcome resistance and broaden the clinical utility of ICB. As an increasing number of treatment options are developed, the concurrent study of patient samples, clinical and radiologic data, and emerging biomarkers will help define which subsets of patients benefit from particular treatments and, equally important, mechanisms of resistance in those who do not. The development of PD-L1 IHC as a companion diagnostic assay and the FDA approval of anti-PD-1 treatment of MSI-H/dMMR tumors demonstrates the ability to use tumor biology for enriching patient responses, sparing patients from ineffective therapy and triaging them to novel therapies.
References available upon request.
Acknowledgments: We thank Joanne E. Tomassini and Sheila Erespe (Merck & Co., Inc., USA) for their writing and editorial assistance.

