Around the Globe
Africa Medicines Agency (AMA) Treaty Update
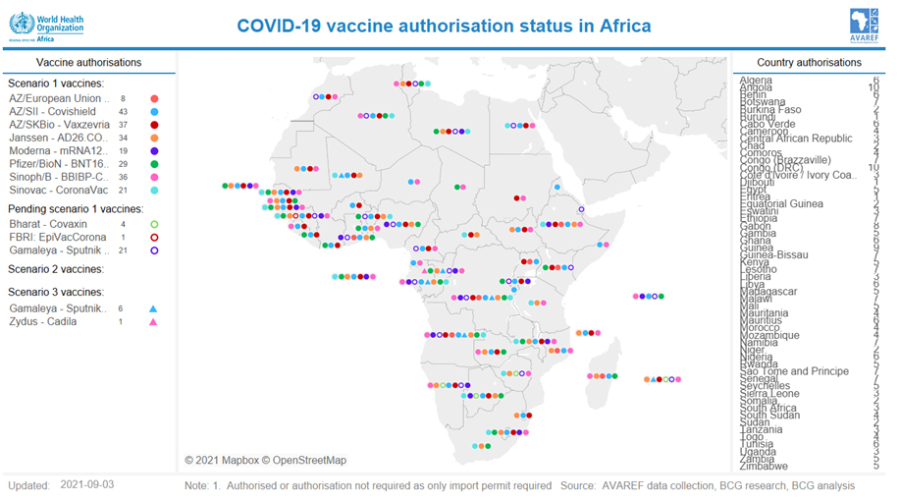
s part of the African Union guidance on emergency regulatory authorization of COVID vaccines, the Africa Vaccine Regulatory Forum (AVAREF) convened several workshops in 2021 with Africa member states (see below). These workshops were intended to enable regulators to rely on the World Health Organization (WHO) Emergency Use Listing (EUL) without having to undertake their own reviews, and in turn accelerate national emergency use authorization and access to these vaccines.
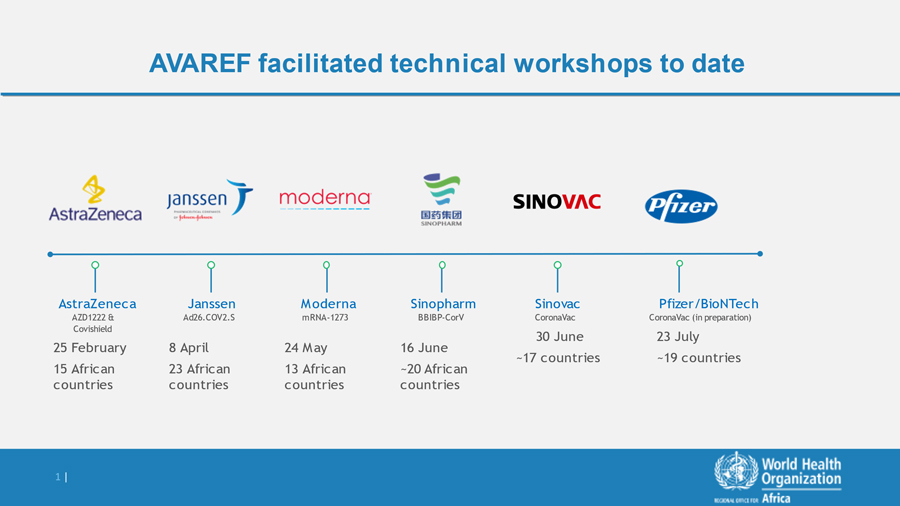
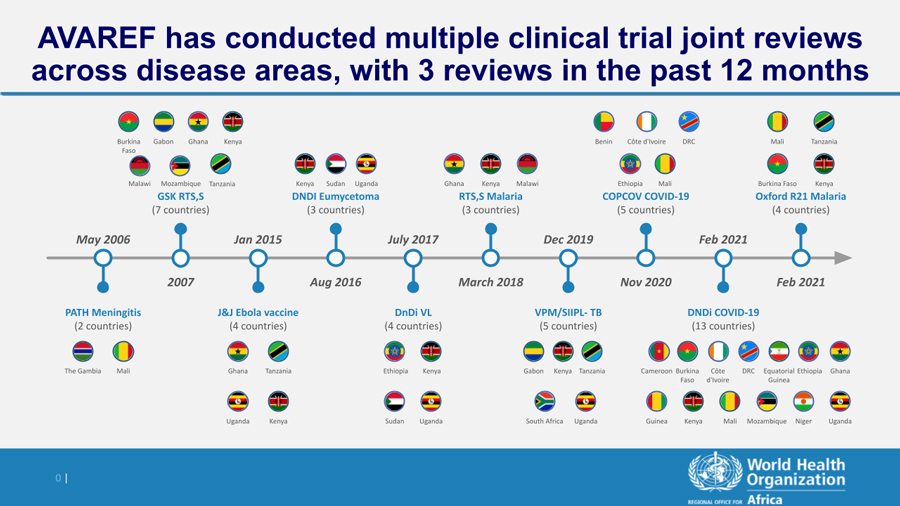
AVAREF Joint Reviews of Clinical Trials
Previous AVAREF facilitated joint reviews are shown below:
Africa Medicines Agency (AMA) Treaty
The AU and WHO are working together on the preparatory phase for establishing the AMA, including where it will be hosted, identifying the inaugural director general and secretariat staff, and defining business processes, funding sources, and other details.
We could see the AMA open its doors within the next year. This is simply amazing given how quickly things have moved since the AMA was endorsed to start the treaty ratification journey at the Heads of State Summit in 2019. Honorable Michele Sidibé and the AU have done a commendable job in driving the ratification process during the past year. We should all embrace the AMA and support Africa as it establishes this agency that will transform in monumental ways the continent’s regulatory ecosystem once established.
A more detailed article on AMA will follow in a subsequent issue of Global Forum.

