Around the Globe
NMPA Moves ICH E2 Forward in China
Key Points of the “Good Safety Information Evaluation and Management Practice during Drug Clinical Trials”
DeltaMed
ver the past two years, the Chinese National Medical Products Administration (NMPA) has aggressively implemented a series of ICH E2 guidances. On July 1, 2020, the Center for Drug Evaluation (CDE) of NMPA issued the “Good Safety Information Evaluation and Management Practice during Drug Clinical Trials” (hereinafter referred to as “Practice”); it was effective immediately on a trial basis.
Based on the “Drug Administration Law,” “Drug Registration,” and “Good Clinical Practice (GCP)” regulations, the “Practice” elaborates on four aspects of an IND Applicant’s responsibilities during a clinical trial:
- Establish a good pharmacovigilance (PV) practice system to evaluate and manage risk.
- Conduct risk monitoring, identification, and evaluation, and proactively take necessary risk control and risk minimization action.
- Comply with risk assessment and management process established by the regulatory authority.
- Promptly communicate all safety risk information to all stakeholders, including CDE.
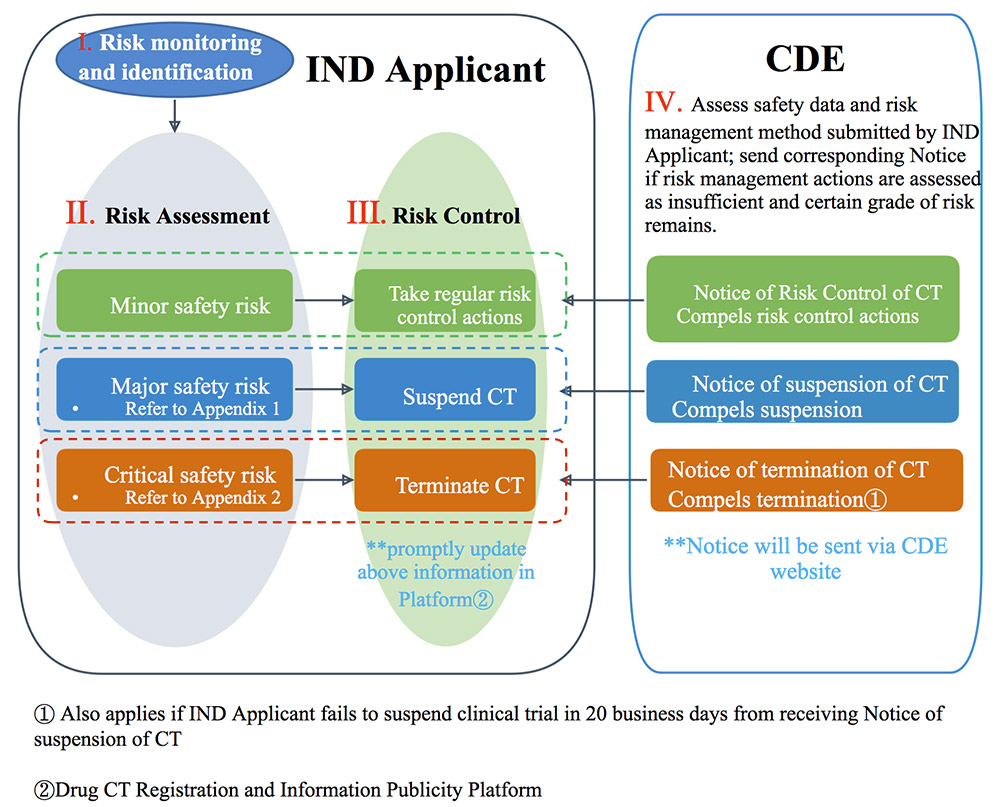
The “Practice” reinforces the fact that the IND Applicant should submit individual SUSARs and other potentially significant safety information in a timely fashion according to the “Standards and Procedures for Expedited Reporting of Safety Data during Drug Clinical Trials” issued by CDE, and submit DSURs annually according to the “Good Management Practice for Development Safety Update Report.” As part of the obligations for safety risk management during clinical trials, the IND sponsor should assess and categorize the safety risks into “Minor Safety Risk,” “Major Safety Risk,” and “Critical Safety Risk,” and then take risk control and risk minimization actions accordingly and promptly. The CDE may require the IND sponsor to take further risk management actions based on the safety assessment and risk management actions submitted by the IND sponsor. Further risk management actions include amending the protocol, the Investigator’s Brochure, and the Informed Consent Form, and/or adjusting the reporting period for DSUR, etc. The CDE may eventually force a suspension or termination of the clinical trial if critical safety risks are found to exist, based on CDE evaluations and decisions.
The steps in the risk assessment and management procedure of the IND sponsor and the regulatory authority are summarized below.