Around The Globe
Japan’s Role in Promoting Global Clinical Research
The Model of Osaka University Hospital
Daisaku Nakatani
Chisa Tabata
Masato Shiren
Koji Nishida
Akira Myoui
Tomomi Yamada
Yukio Tanaka
Yoichi Yamamoto
Bolrathanak Oeun
Hideaki Harada
Ken Nakata
Junzo Seki
Mayuko Ouchi
Department of Medical Innovation
Osaka University Hospital
Japan’s Role in Promoting Global Clinical Research
The Model of Osaka University Hospital
Daisaku Nakatani
Chisa Tabata
Masato Shiren
Koji Nishida
Akira Myoui
Tomomi Yamada
Yukio Tanaka
Yoichi Yamamoto
Bolrathanak Oeun
Hideaki Harada
Ken Nakata
Junzo Seki
Mayuko Ouchi
Department of Medical Innovation
Osaka University Hospital
he number of industry-initiated global clinical trials conducted simultaneously in multiple countries under the same protocol has been increasing annually. This may contribute to avoiding a drug- and device-lag for clinical use across the world. However, we must recognize that the differences in regulations, standards of treatment, and medical insurance systems in different countries, as well as ethnic factors, may lead to challenges for the conduct of clinical trials.
In October 2016, Osaka University Hospital was awarded the Global Clinical Trial Development Project led by the Japan Agency for Medical Research and Development (AMED). In response to this award, the Global Clinical Research Support Group (GCRSG) was launched within the Department of Medical Innovation (DMI). The Group was set up independently of the four existing centers in the DMI in order to receive support from all centers. In April 2018, the GCRSG was moved under DMI’s Center for Global Health (Figure 1). The GCRSG aims to respond to and support a range of activities, including planning and designing multinational clinical research and trials, and regulatory actions and negotiations from the perspective of future global development in collaboration with other clinical research core hospitals, thereby realizing the mission that multinational clinical research and trials initiated by physicians in Japan in particular should play a central role in such global activities.
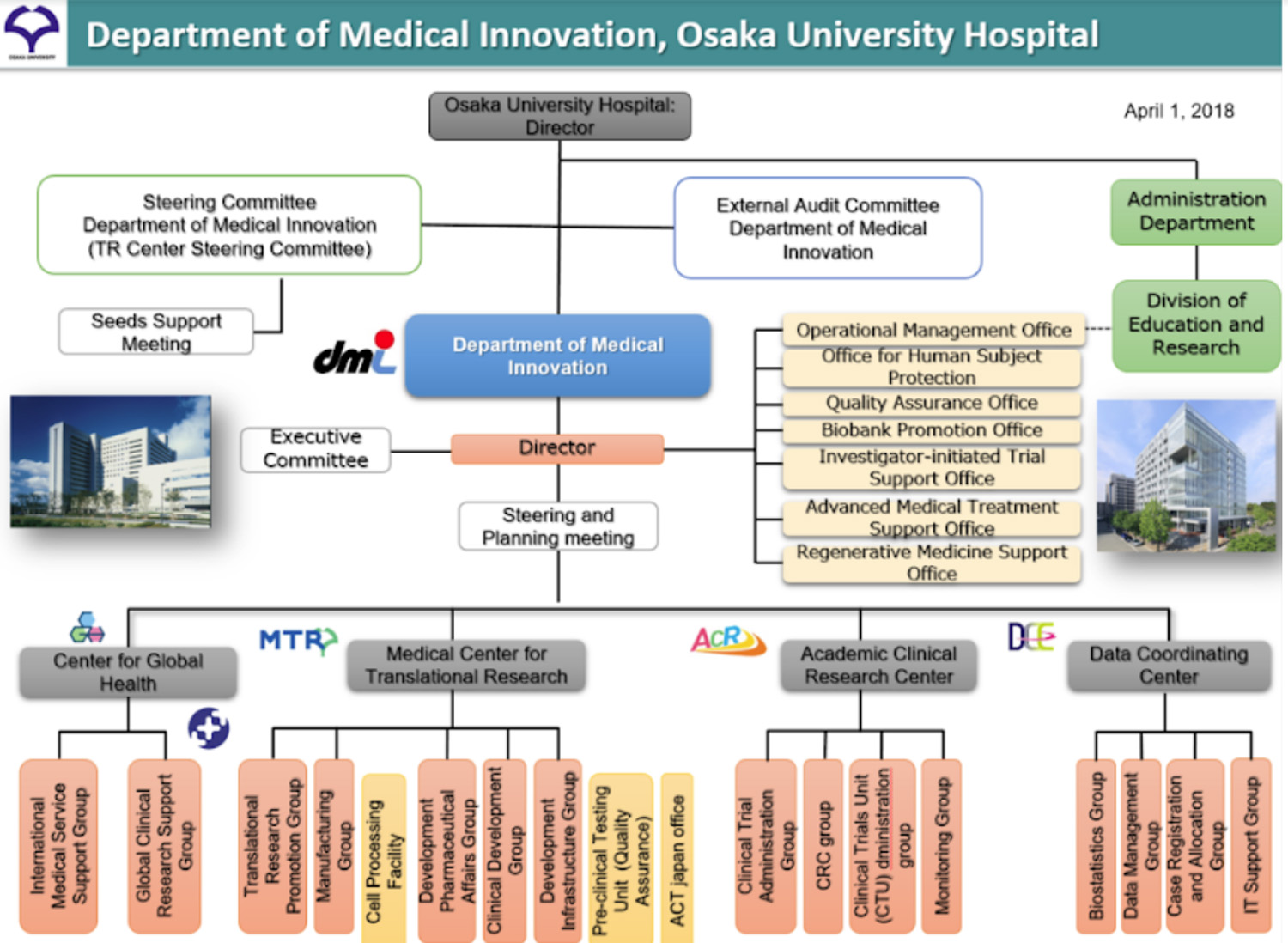
Figure 1. Organization of Department of Medical Innovation, Osaka University Hospital
The GCRSG has organized a Japanese consortium of clinical research core hospitals (J-CCRC), consisting of 12 clinical core hospitals across Japan, certified to conduct this research by the Ministry of Health Labor and Welfare because they fulfill specific criteria, such as compliance with ICH-GCP and appropriate ethics review of clinical trials. The aim of the J-CCRC is to share issues that must be resolved while conducting global academic clinical research.
To date, issues we have shared include 1) distribution of grants from the Japanese government to other countries, 2) difficulties in developing protocols that account for country-specific differences, and 3) listing authors located in different countries on scientific articles. These issues need to be resolved patiently through negotiations with investigators in all involved countries before their collaborative work can start. Another aim is to collaborate with other consortia in various countries, which may help investigators more easily find appropriate institutions to conduct global clinical research.
As of August 2018, the GCRSG has supported 14 global clinical studies (Table 1). Of those, six are from investigators at Osaka University Hospital, and eight are from researchers outside of the hospitals in Japan. Unlike clinical research conducted by industry, the global clinical research focus in academia may include areas in which pharmaceutical companies are not interested, such as rare diseases, tropical infectious diseases, and surgical techniques. The drugs, devices, and regenerative medicines that are used in these projects have not yet been approved. Our main goal is to support regulatory approval for new drugs, devices, and regenerative medicine.
Table 1. Global projects supported by the Global Clinical Research Support Group: Internal projects were seeded by Osaka University Hospital; external projects were seeded by hospitals outside GCRSG core clinical research hospital network.
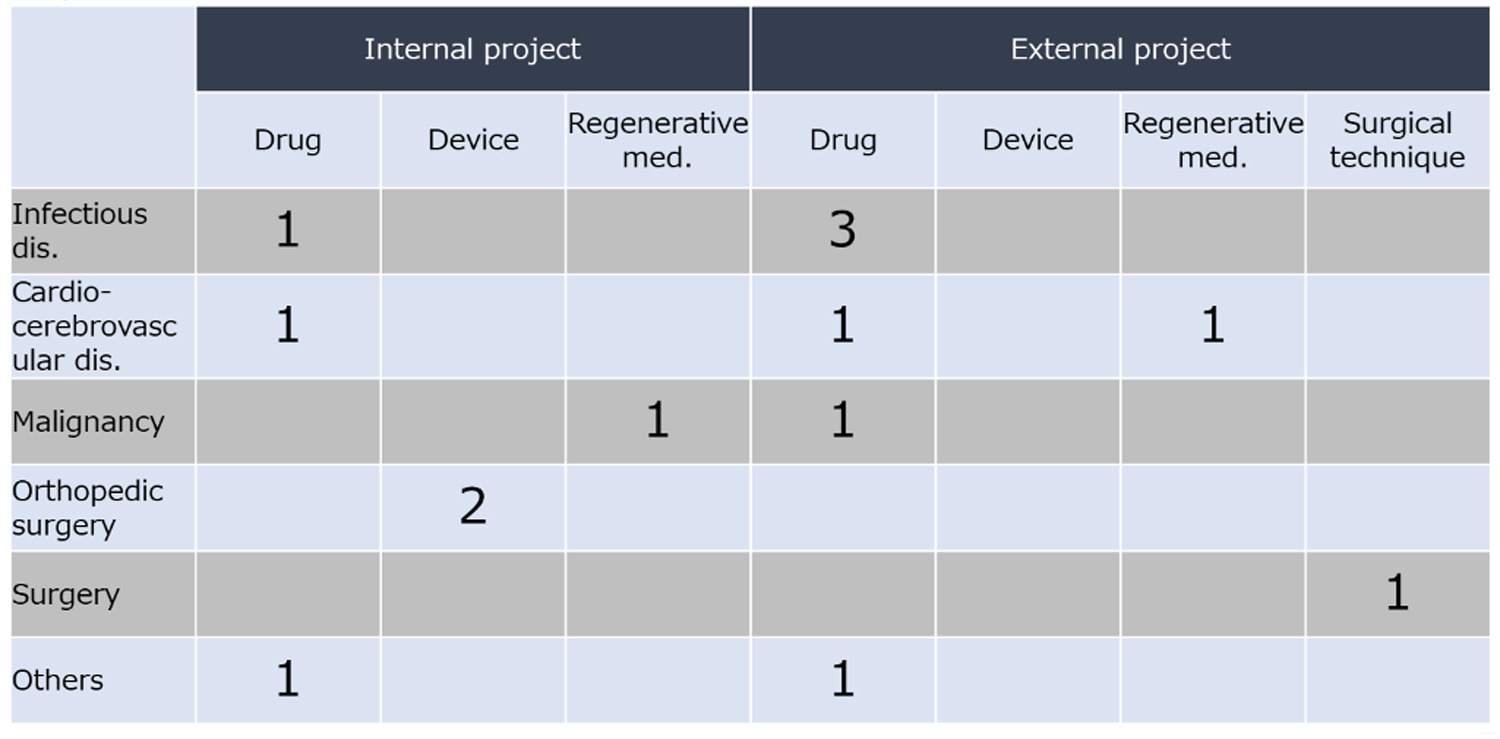
The four infectious disease projects we are supporting include one for Neglected Tropical Diseases (NTDs); one for viral infectious diseases; and two addressing one of the three major infectious diseases (Human Immunodeficiency Virus (HIV), tuberculosis and malaria). One of these four projects has already started successfully in a West African country; the other three will be conducted soon, also in African countries.
We also support global registry studies in order to explore and collect clinical data on patient characteristics, treatments, and outcomes, before starting clinical trials. This two-tier strategy may help develop more adequate protocols and better facilitate recruiting patients from these registries for global clinical trials that require regulatory approval.
We believe that our efforts will help facilitate global clinical research and ultimately result in regulatory approval for new drugs, devices, and regenerative therapies originating in academia. In addition to facilitating regulatory approval of products, the GCRSG is engaged in efforts to improve other aspects of patient care. In some cases, a project does not aim to get regulatory approval but to change a guideline. For example, a surgical project aims to explore the efficacy and safety of a surgical technique. The final goal of this project, if the results are positive, is to update the relevant guideline. Our website further describes our efforts to support global clinical research. Our website further describes our efforts to support global clinical research.
Acknowledgements: The authors thank Rika Guzman, Aya Mori, and Natsuha Yoshida for their excellent assistance as secretariats.

