Survey Results: Improving Clinical Research Literacy Among Underserved Communities
The Center for Information and Study on Clinical Research Participation (CISCRP)
CISCRP
Tufts Center for the Study of Drug Development, Tufts University School of Medicine
t is essential that representative communities—including patients from medically underserved communities—participate in clinical research. Clinical trial data from diverse populations play a vital role in translating scientific advancements into better health outcomes for all the people who may benefit from a new therapy. However, clinical research literacy and levels of trust vary widely, in part because the general public and patients have limited exposure and access to clinical research, and they may consider it to be of limited relevance in relation to their health and the community where they affiliate and live.
Several months in advance of the mobile exhibit’s event dates, CISCRP partnered with six local community educators in Baltimore and Philadelphia with the goals of establishing lasting connections in underserved communities and increasing attendance at the mobile exhibit. Community educators were compensated for their time.
A recreational vehicle (RV) (Exhibit 1) was outfitted with an interactive educational exhibit about clinical research. In the fall of 2023, the mobile exhibit was brought to six major events in underserved communities, three in Baltimore and three in Philadelphia.
At each event, community educators and CISCRP staff welcomed people passing by and guided them through the exhibit and facilitated conversations afterward. Surveys were conducted before and after exhibit visits to evaluate the impact of the exhibit on visitors’ attitudes and behaviors regarding clinical research.

In order to provide visitors with background on reasons for underrepresentation and mistrust in clinical trials, exhibit materials discussed examples of past injustices and mistreatments of those who are Black or African American as well as those who are Puerto Rican, specifically historical events such as the Tuskegee Experiment, the mistreatment of Henrietta Lacks, and unethical contraceptive trials conducted in Puerto Rico.
The exhibit also discussed regulations and safeguards in the current clinical research landscape (e.g., an overview of how the safety and ethics of clinical trials are monitored by IRBs and the FDA), provided information about the importance of clinical research, and described ways to get involved. The information highlighted the importance of diverse representation in all aspects of the clinical research process, including on patient advisory boards and in clinical trials.
Assessing Impact
Three short surveys were developed to assess visitors’ attitudes toward clinical trials immediately before (Survey 1), immediately after (Survey 2), and one month after they visited the exhibit (Survey 3).
Respondent Characteristics
Of the 676 people who visited the JTBH mobile exhibit, more than half (n=355) completed both Surveys 1 and 2, while 103 of those visitors also completed Survey 3.
Almost half of those who disclosed their race and ethnicity (n=156, 49%) identified as Black/African American, 10% (n=31) identified as Asian, 17% (n=53) were of Hispanic or Latino ethnic origin, and 38% (n=122) identified as White. Over half of survey respondents were between 18 and 34 years of age (n=207, 58%) with a majority identifying as female (n=236, 68%). Note: Values may not add to 100% because not all respondents disclosed their demographic information, and respondents could select multiple values for some demographics questions.
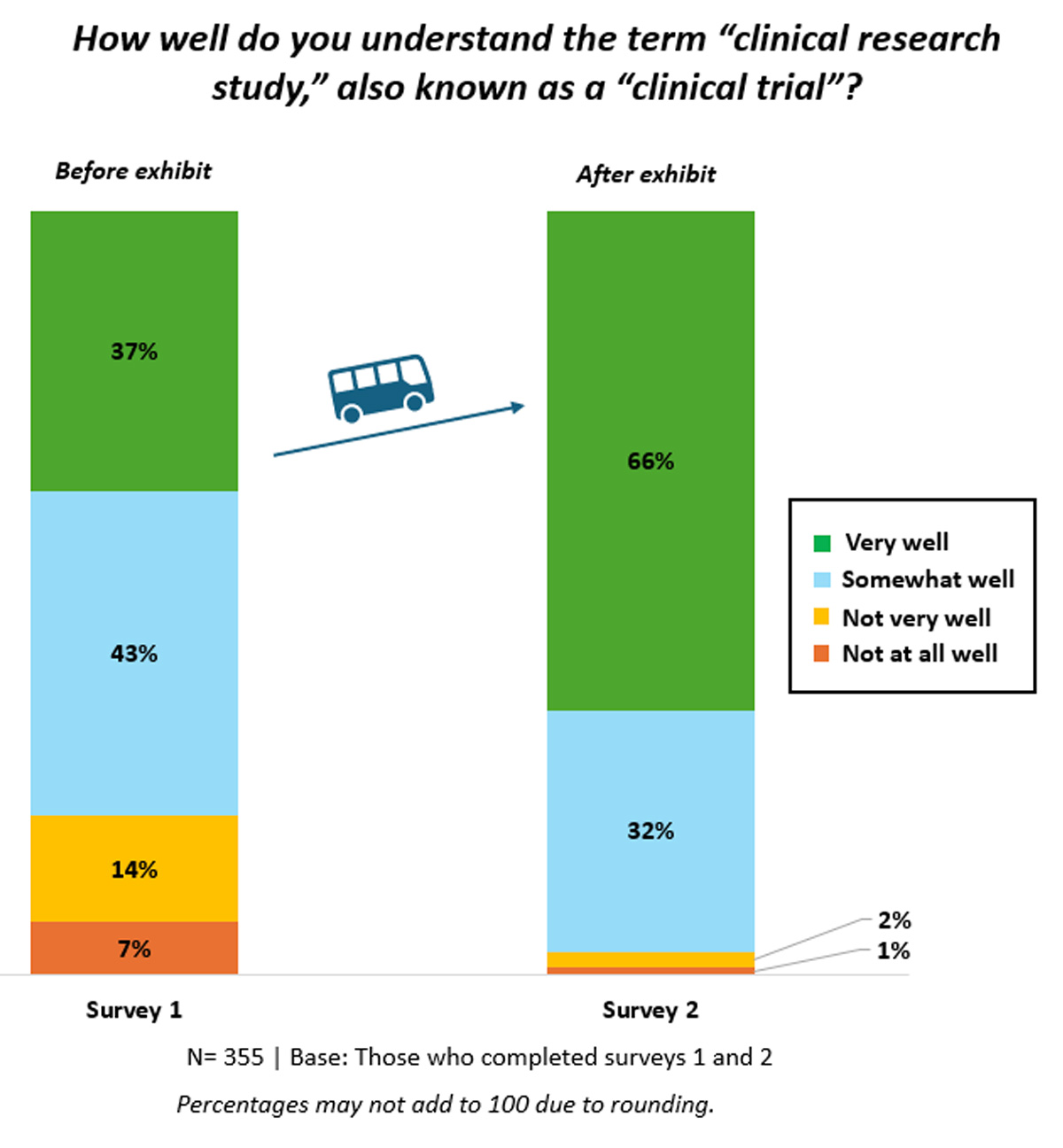
The mobile exhibit positively impacted visitors’ general understanding of clinical research. Prior to experiencing the mobile exhibit (Survey 1), 37% (n=132) of respondents reported having a strong understanding of the terms “clinical research study” and “clinical trial,” while this number rose to 66% (n=233) immediately after visiting the mobile exhibit (Figure 1).
When visitors were asked (in Survey 2) about their experience visiting the mobile exhibit, responses were overwhelmingly positive. The majority of participants (68%) reported their experience as “excellent” or “very good” (28%) (n=355).
Discussion
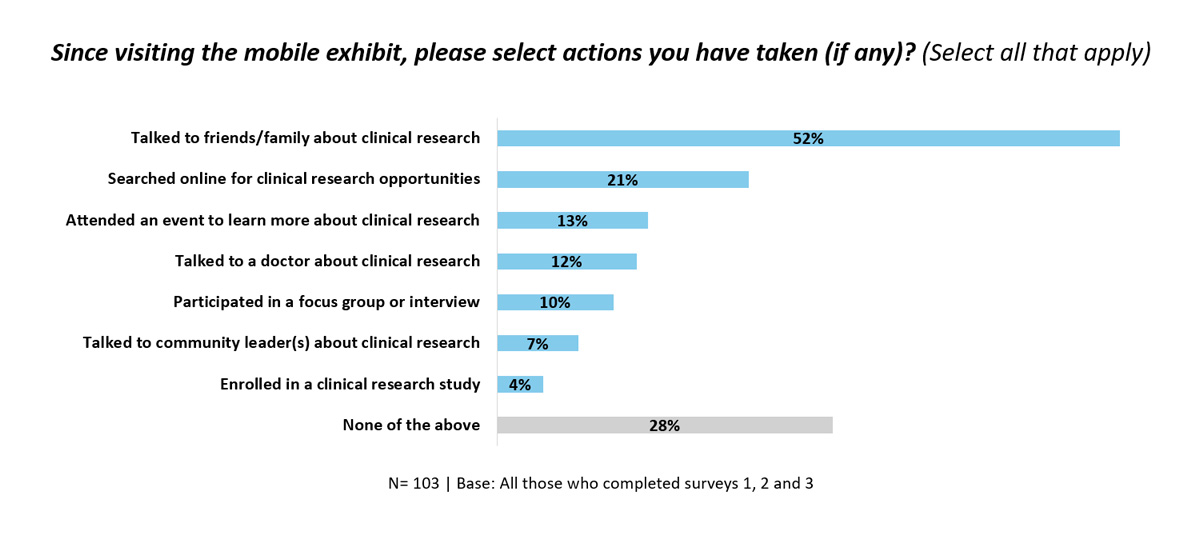
The results of the JTBH mobile exhibit impact surveys suggest that this novel, mobile model of educational outreach may be an effective method of raising awareness of, and boosting public engagement in, the clinical research process among medically underserved communities. Results also showed that this educational exhibit model may present a step in the right direction toward increasing awareness of the importance of representation in clinical research and may lay a foundation for long-term engagement in the clinical research process.
CISCRP will be bringing, and assessing the impact of, the mobile exhibit to rural underserved communities in late 2024 and into 2025.
Acknowledgements
We thank the community educators, volunteers, and CISCRP staff whose hard work and dedication made this project possible.
This project is supported by the Food and Drug Administration (FDA) Office of Minority Health and Health Equity (OMHHE) of the US Department of Health and Human Services (HHS) as part of a financial assistance award (FAIN). The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement by, FDA/HHS, or the US government.

