Around the Globe
Japan Association for the Advancement of Medical Equipment
(JAAME)
n December 2018, the first AI-based medical device–the AI software to support discrimination between tumor/nontumor colorectal lesions using endoscopic images–was approved in Japan. Although nearly three years have passed since then, the number of AI-based medical devices approved in Japan is still low compared to Europe and the US.
It is important to consider these three aspects when examining policies related to medical devices: research and development, regulatory aspects of efficacy and safety, and reimbursement pricing. Japan began studying these three points in January 2017 and, after continuous discussions, the cabinet approved the Regulatory Reform Implementation Plan in June 2021. This plan summarized various policies to address most of the issues to be tackled in the future.
Relevant initiatives in this journey from study to implementation plan are overviewed below.
June 2017: Ministry of Health, Labor and Welfare (MHLW) Round-Table Conference on Promoting the Use of AI in Healthcare
The report from the Round-Table Conference on Promoting the Use of AI in Healthcare identified six priority areas to be developed for AI utilization in healthcare and formulated a roadmap to 2020 for their realization: 1) diagnostic imaging support, 2) diagnostic and therapeutic support (including testing, disease management, and disease prevention), 3) surgical support, 4) nursing care and dementia, 5) genomic medicine, and 6) drug development.
December 2017: PMDA Scientific Committee Recommendations on Review of AI-Based Medical Devices
March 2019: Draft Review Guidance for AI Medical Devices (MHLW, NIHS)
June 2019: Consortium for Accelerating AI Development in Healthcare Identifies Roadblocks by Development Stage (MHLW)
November 2019: Amendment of Pharmaceuticals and Medical Devices Act (PMD Act) to Establish PACMP
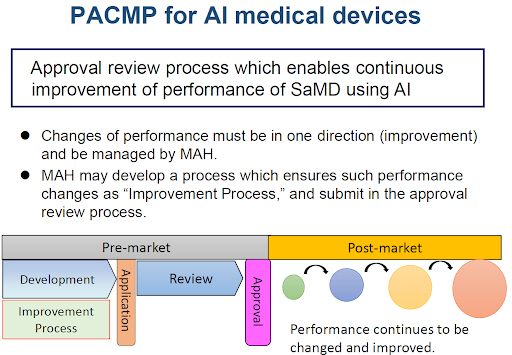
SaMD is an acronym for Software as a Medical Device.
June 2020: Consortium for Accelerating AI Development in Healthcare Establishes Process Chart for Resolving Roadblocks (MHLW)
To help facilitate the introduction of AI-based medical devices into clinical practice in Japan, the MHLW published a roadmap for the elimination of roadblocks in each of the nine stages of development until FY2022. MHLW is currently implementing various measures to resolve issues according to this roadmap.
November 2020: DASH for SaMD to be Developed (MHLW)
March 2021: Start of Discussions to Clarify Concept of SaMD in Reimbursement Pricing
June 2021: Regulatory Reform Implementation Plan for Japan
Conclusions
In a related effort, the Roundtable Conference on the Promotion of Venture Businesses for Medical Innovation report compiled by a private panel of the MHLW in July 2016 established three principles for specific measures to promote medical business ventures: “from regulation to cultivation,” “from caution to speed,” and “from macro to micro.”
Since then, the overall policy goal for innovative medical product review and approval has changed from “catching up” (to the US and Europe) to providing an “incubation function” to help industry provide patients with cutting-edge medical technologies as quickly as possible, before other nations instead of after. Japan has developed a practical approach to AI-based medical devices, decided on priority areas, created a roadmap for their realization, and is implementing a five-year program in which industry, academia, and government work together to identify and resolve roadblocks to these efforts.
These are some of the reasons why my answer to the opening question is Yes.

