Around the Globe
early two years have passed since we began to face the COVID-19 pandemic, and while its global consequences have been catastrophic, the situation has also catalyzed profound change in our overall health ecosystem. The progress we have experienced in drug development, transformative therapeutic innovation, and regulatory cooperation and harmonization continues to spread. Global health awareness has grown significantly, and global stakeholders continue to focus on exploring new areas of health science and technology for innovation, framing regulatory strategies and policies, and developing new leadership for this changing healthcare world.
Each year, millions of patients choose flu vaccines delivered via the nose. The speed of vaccination can exponentially increase in intranasal vaccines, and even self-administration is a safe option.
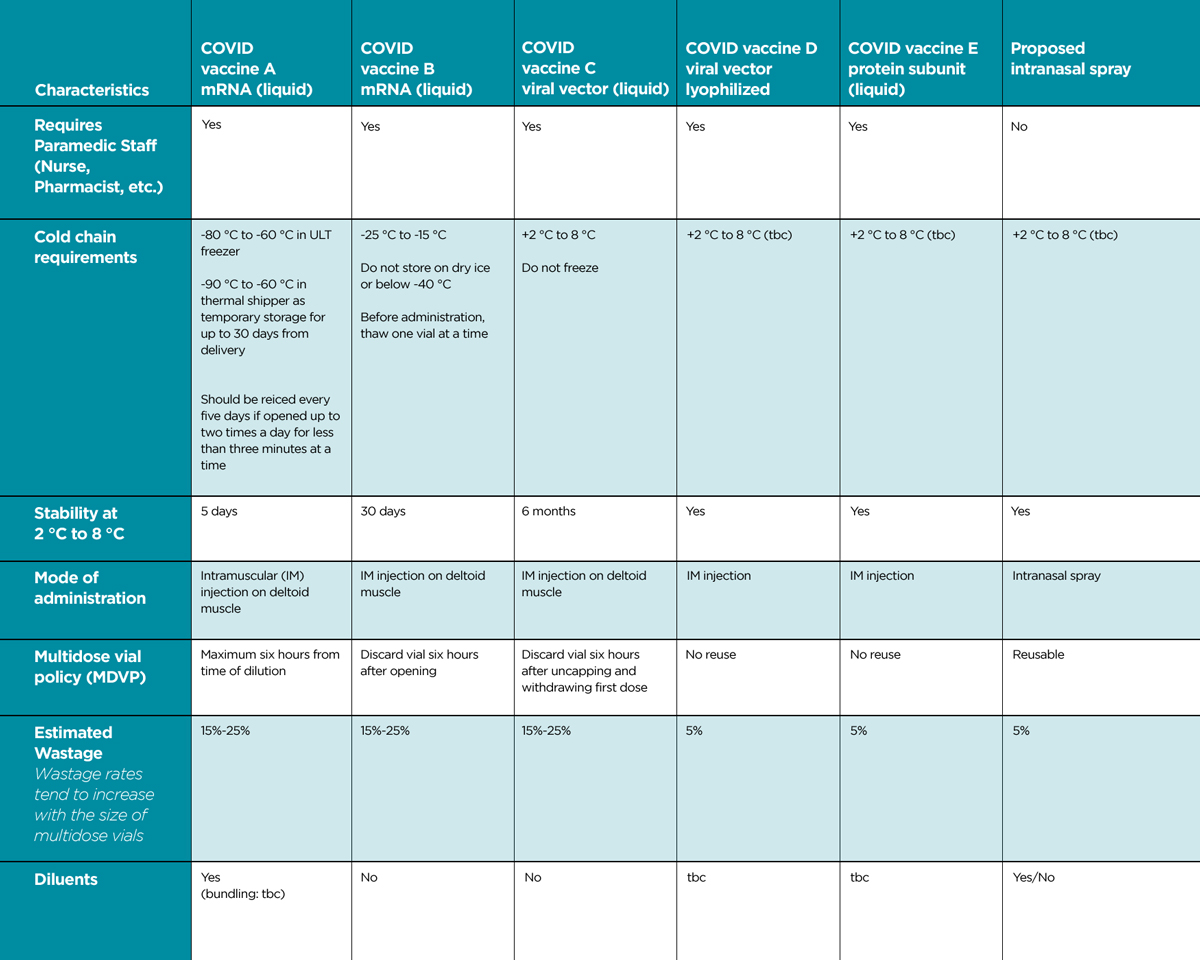
Three COVID-19 vaccines are authorized for use in the US, each with varying storage temperature requirements. In addition, vaccines often come in multidose vials to realize efficiencies from packaging multiple doses in a single vial; however, this can also result in wastage.
Other logistical challenges include potential long lead times spent preparing, installing, and deploying sites for large-format cold storage (e.g., power access, sufficient floor space, security/access), and a lack of long-term utility for large-format cold storage (i.e., repurposing for post-COVID-19). Table 1 summarizes some of the characteristics and potential advantages/disadvantages of injectable versus inhaled intranasal vaccines.
It has also been observed that a high percentage of vaccines must be discarded due to failure in the cold chain (“wastage”). In a 2005 paper, the World Health Organization (WHO) reported global vaccine wastage at more than 50%, a number still cited by the United Nations Environment Programme. Recently, the French Directorate General of Health has stated that it is operating with the cautious estimate of a 30% wastage rate on COVID-19 vaccines distributed in the country.
One advantage for the intranasal route is that it can use liquid and dry powder formulations. The latter should provide clear advantages in transportation, storage, and wastage because a cold chain may no longer be required. In this respect, the superior thermostability of intranasal vaccine would be a breakthrough.
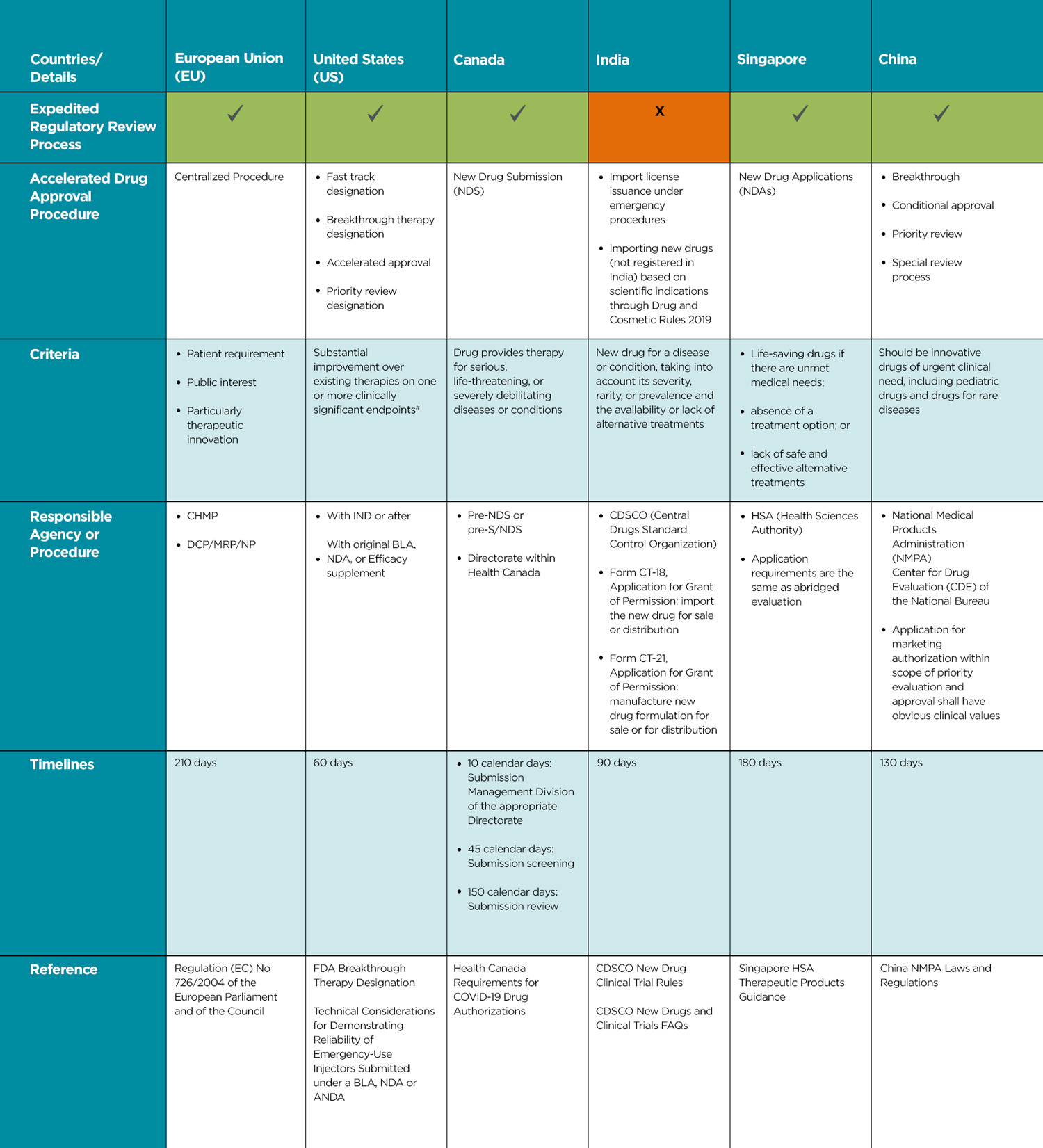
Global Accelerated Regulatory Pathways
Table 2 summarizes the state of accelerated or expedited regulatory approval pathways in various locations.
Reference:
European Union (EU) – Regulation (EC) No 726/2004 of the European Parliament and of the Council
United States (US) – FDA Breakthrough Therapy Designation, Technical Considerations for Demonstrating Reliability of Emergency-Use Injectors Submitted under a BLA, NDA or ANDA
Canada – Health Canada Requirements for COVID-19 Drug Authorizations
India – CDSCO New Drug Clinical Trial Rules, CDSCO New Drugs and Clinical Trials FAQs
Singapore – Singapore HSA Therapeutic Products Guidance
China – China NMPA Laws and Regulations
CHMP: Committee for Medicinal Products for Human Use
DCP/MRP/NP: Decentralized/Mutual Recognition/National Procedure
# Details under Guidance for Expedited programs for serious conditions – Drugs and Biologics
Conclusion
- Pediatric patients: Easier to use, noninvasive
- Elderly patients: Easier to use, noninvasive
- HIV-infected patients: Reduced anxiety of needle stick injury or infection
- Multimorbidity patients: Reduced number of injections and associated anxiety.
Advances in drug delivery, including connecting intranasal vaccine administration to tracking or other medical devices, may present alternatives to multiple injections and help meet patient needs during the challenging and often diverse conditions presented by COVID-19.
References available upon request.
The authors thank Janine Jamieson for support, discussions, and guidance on combination products.