Around the Globe
Best Practices from Cuba and Mexico
Centre for Innovation in Regulatory Science (CIRS)
IFAPP Academy
Wessx Consulting
he COVID pandemic precipitated sweeping changes in established policies and practices and emphasized the confluent dynamics of disease burdens, optimized healthcare systems, and the socio-economic influences of globalization. We now stand at the crossroads of an unprecedented global challenge which demands that researchers, regulators, policy makers, and governments address multiple dimensions that go far beyond the implications of any one healthcare scenario.
As the healthcare and regulatory landscape evolves in Latin America, early dialogue and enhanced partnerships between biopharmaceutical industry stakeholders, and streamlined coordination among healthcare and regulatory agencies, are critical to expedite decision-making, promote increasingly sophisticated systems and data-sharing, and leverage global ecosystems and synergies in response to emerging regional challenges.
Fostering innovation, translating learnings into competency-based training and education, prioritizing resources, and optimizing clinical development and regulatory models will not only deliver better medicines to the region (and better value to its patients) but also help Latin America reimagine sustainable solutions for unmet medical and social needs, and remain relevant on the global stage.
In parallel, it is paramount to continue to cultivate trust and transparency.
Regulatory Modernization: Best Practices from Cuba and Mexico
Enabling a regulatory system that looks forward and continually evolves to support innovative product research and regulation is critical. Three ways that an agency can work toward these goals are by implementing regulatory science best practices; following international standards; and establishing an Innovation Office to coordinate these activities.
The Cuban regulatory agency (CECMED) has recently addressed this need through developing its Office of Innovation with the aim of building a comprehensive regulatory framework to accelerate innovation and enable the transition of innovative products from clinical research to clinical practice—the first formal initiative of this type in Latin America and the Caribbean. Its goal is to serve a leading role in national and regional biopharmaceutical innovation by adopting science-based approaches and regulatory strategies for product development by improving the flow of knowledge across drug innovators, regulatory agencies, and regulatory scientific committees. While the establishment of this office in CECMED contributes to the efficiency and productivity of the national Cuban biotechnology industry, the office will also play an international role as CECMED seeks to align its harmonization initiatives with the European innovation networks and regulations to ensure better alignment for new market access opportunities for Cuban biotechnology products.
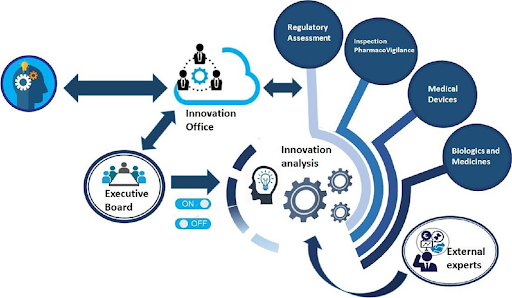
Similarly, the Mexican Medicines Regulatory Agency (COFEPRIS) has developed and implemented a unique scheme to support innovative research and product regulation through its Center for Innovation and Training, which was inaugurated in March 2021 and has already transformed communications with its stakeholders. The center’s main objectives are to:
- Lead a horizontal innovation strategy throughout COFEPRIS.
- Revitalize communication so that scientific knowledge, ideas, and experiences are available through an exchange platform in a transparent, open, and direct manner.
- Transform the concept of public health innovation: taking initiatives into the real world.
Initiatives currently supported by this center include:
EducaPRIS: Weekly training videoconferences focused on procedures and requirements for regulations, and the vehicle that informs about operational and regulatory changes to optimize the stakeholder’s experience.
InnovaPRIS: Monthly virtual dialogue space where academics, research centers, and social organizations exchange ideas and spread innovative practices across regulated industries.
Ciencia Cofepris: A periodic publication aiming to widely disseminate scientific knowledge about preventing health risks. The first issue addressed planetary health and global solidarity.
Regulatory Innovation Working Groups: Joint efforts to improve and develop new regulations; topics considered for study include medical software and orphan drugs.
International Alliances: Such as current alliances with Doctors Without Borders to evaluate antimicrobial resistance in rural areas and with the Drugs for Neglected Diseases Initiative to improve access to these drugs throughout Latin America.
In addition to these initiatives directly linked to the COFEPRIS Center for Innovation and Training, the agency plans to address renewed efforts to expand electronic procedures, continue with international harmonization, and improve its operating efficiency, among other initiatives.
The Way Forward
To leverage these best practices and lessons learned by regulators from Cuba and Mexico, stakeholders in Latin America should consider creating initiatives dedicated to discussing joint solutions that would enable greater access to medical innovation in the region.
This regional “innovation task force” could be designed as a working group which reflects on practical working models that prioritize innovation and enable horizon-scanning of innovative products and identify practical and up-to-date solutions to complex and sometimes threatening real-life healthcare situations, such as pandemics or other outbreaks of potentially deadly diseases.
Such working groups would benefit from current initiatives that convene regulatory authorities in the region, such as the Ibero-American Medicines Authorities Network (EAMI), a regulatory network that could conveniently connect Latin American efforts to the more well-developed innovation landscape in Europe. For instance, regulators and other key stakeholders could collaborate in jointly assessing breakthrough therapies and other novel drug product candidates, pooling resources and sharing technical expertise, as recommended by the World Health Organization’s Good Reliance Practices and Good Regulatory Practices. Stakeholder cooperation, transparency, openness to dialogue, and a mindset favorable to work-sharing can genuinely support scientific regulatory thinking across the region.
Aligning regional stakeholder partnerships, in which joint cooperation and reciprocity are vital elements for maximizing regulatory skills and competencies, is the way to deliver on the promises of access to healthcare innovations in Latin America.

