Regulatory Harmonization in East Africa
The Story, Highlights, and Promise
Bill & Melinda Gates Foundation
n August 12, 2020, PLOS Medicine published a collection of five papers on the East Africa regulatory harmonization initiative. You can find these papers online. This article will provide highlights of this collection.
This collection reports the early adoption of the Common Technical Document (CTD) across the region as well as shared regulatory requirements and standards for medical products regulation; this set the stage for countries to start assessing marketing applications (MAs) jointly. The initiative also enabled, through strong advocacy, creation of new autonomous regulatory agencies in Rwanda, South Sudan, and Zanzibar.
Between 2015 and 2019, the regional joint reviews assessed 83 medicinal products, resulting in the recommendation of 36 products for registration. Overall, the joint assessment median timeline, from submission of the application through final decision, was a little more than one year (372 days); 170 of these days represent time used by manufacturers to answer queries. However, the median timeline for a joint assessment in 2019 was only 240 days, indicating that the process had become more efficient. Regulatory timelines for applications submitted through the national route also dropped to 18-24 months compared to the 24-28 months reported in 2012.
Partners involved in this initiative include the African Union, the World Bank, World Health Organization, Swiss Agency for Therapeutic Products (Swissmedic), United Kingdom Department for International Development, and Bill & Melinda Gates Foundation, who provided the needed technical, financial, and political advocacy support.
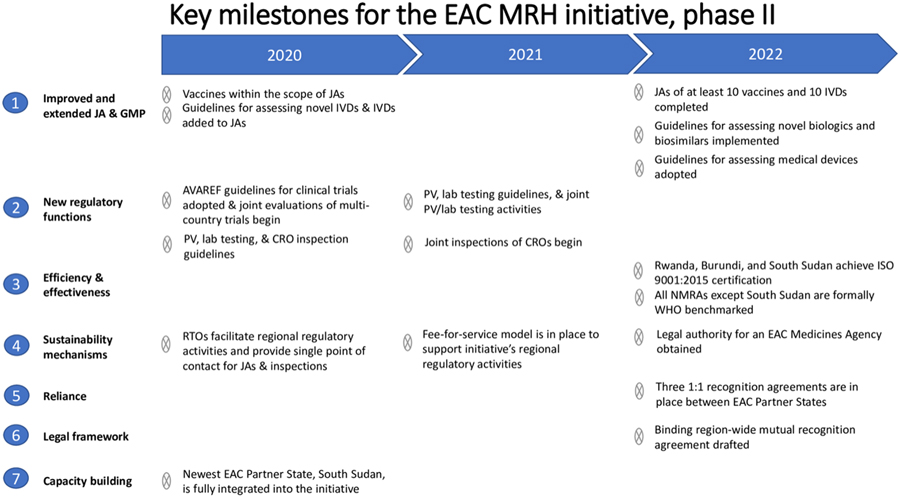
Summary Descriptions
Paper 2 establishes the initiative’s foundation, rationale, and history. It describes EAC’s aspirations to increase the number of quality medicines it registers by simplifying the application process for manufacturers. EAC also aims to increase the speed at which it reviews applications by modernizing assessment processes and procedures, without decreasing scientific rigor.
Paper 3 reports on the design and implementation of the initiative as well as key results or outcomes. The initiative’s initial focus was on registering generic medicines, with a plan to expand to other classes of medical products, as well as to other regulatory functions. This paper also discusses challenges, such as reliance on donor support, and how to become a self-sustaining program and a permanent feature of the African regulatory ecosystem.
Paper 4 describes the EAC roadmap for the future, which includes expansion to other regulatory functions, as well as how the initiative will address gaps and challenges identified in the initial phase. The roadmap is expected to lead to a stronger, more efficient, and more accountable region-wide regulatory system.
- The goal of improving access to quality medicines in East Africa has been addressed by the initiative, which is working to simplify the process of registering medicines and increase the speed at which registration applications are reviewed, while ensuring that only high-quality medicines are approved.
- The rapid implementation of a joint product assessment process through which a manufacturer can submit a single marketing authorization application and receive a decision respected by all EAC member states, as well as the creation and use of a common technical document for registration applications that is accepted by all member states, are strengths of the initiative.
- Strengthen the legal framework, so that member states are legally required to respect joint regulatory decisions instead of being bound only by goodwill.
- Other suggestions for moving forward include: Address increasing transparency, inviting and responding to feedback from industry partners, consistently meeting its advertised assessment and registration timelines, streamlining the joint product assessment process, taking advantage of work done by peer institutions, focusing on the activities most likely to have the greatest public health return, and using metrics and benchmarks to identify opportunities for greater efficiency.
Does This Initiative Have a Future?
Call to Action
Let us not forget that it took Europe several decades for its regulatory system to get to where it is today. Let us support and give the EAC a chance but also hold them to these commitments. As we say at the Gates Foundation: “We are impatient optimists.”

