Patient Focused Medicines Development: The Book of Good Practices
Evolution of Methodology
PFMD/The Synergist
NIHR INVOLVE, UK
PFMD/The Synergist
School of Health Sciences
University of East Anglia, UK
Patient Advocate, Consultant, and Researcher
Medical Innovation and Insights
Pierre Fabre Medicament
PFMD/ The Synergist
Janssen
atient Focused Medicines Development (PFMD), today a global coalition of 32 members, began its Framework Building workstream in 2016 in order to respond to a need for a practical and actionable framework to define how stakeholders can start their patient engagement (PE) journey and continue to do more effective and meaningful PE. Starting with a landscape analysis of existing PE frameworks, one of the first outputs—the Patient Engagement Quality Guidance (PEQG)—was a co-creation effort of multiple Working Groups that involved more than 100 experts globally (representing more than 51 organisations). The PEQG went through four feedback and validation rounds, from internal reviews to a public consultation, before its release in May 2018.
This article explains the methodology used to select and assess initiatives to become a part of the BOGP, and it provides examples of the chosen initiatives that help illustrate what a “good practice” in PE could look like.
The first edition of the BOGP was released together with the PE Quality Guidance in May 2018 with eight case studies from industry, research, and patient organisations. The second edition of the BOGP, released at the end of October (2019), enriches and complements the initial set of examples and represents the evolving PE ecosystem.
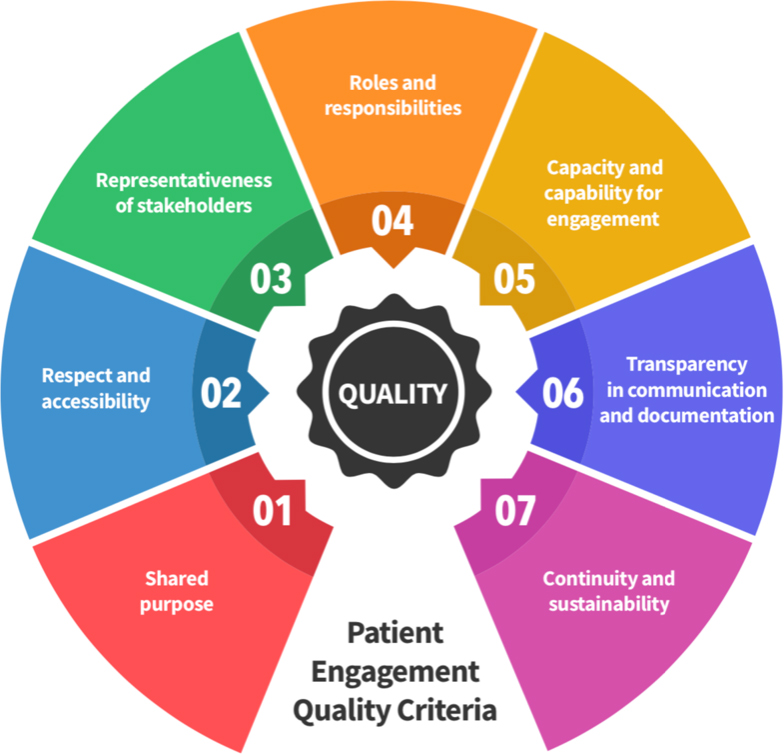
Maintaining the Initial Selection Process While Improving Scoring Methodology
Initial Selection Process by PFMD
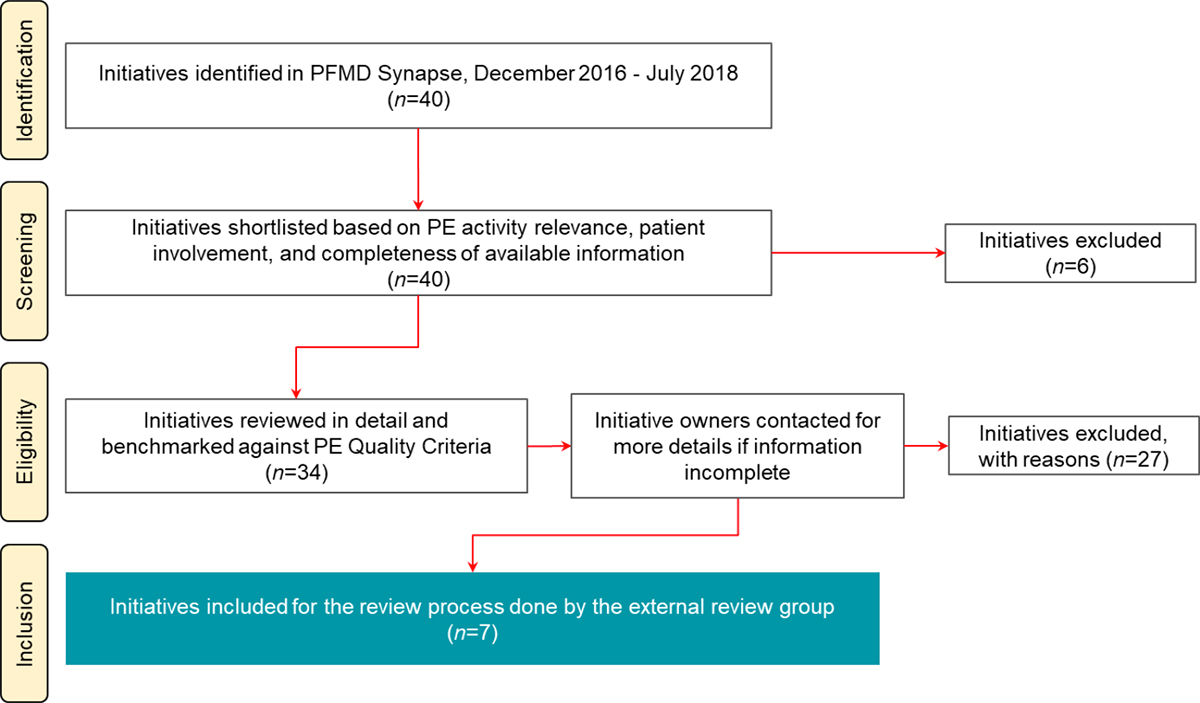
The selection process for the second edition was conducted using the same process as in the first edition of the BOGP (Figure 2):
- Identification: The first step was the identification of initiatives submitted to PFMD via the Synapse database (n=40). This initial selection excluded cases that were already reviewed in the first edition of the BOGP (n=197) and thus showed a smaller selection to begin with.
- Screening: First-pass screening and shortlisting was carried out based on completeness of information on the initiative and on the involvement of multiple stakeholders, one of which needed to be a patient or patient organisation.
- Eligibility: PFMD carried out the first review based on the information from the submissions and requested additional details from initiative owners.
- Inclusion: The remaining initiatives that entered the last stage went through an enhanced scoring process by the external review group to identify and reach consensus on cases for inclusion in the BOGP, as explained below.
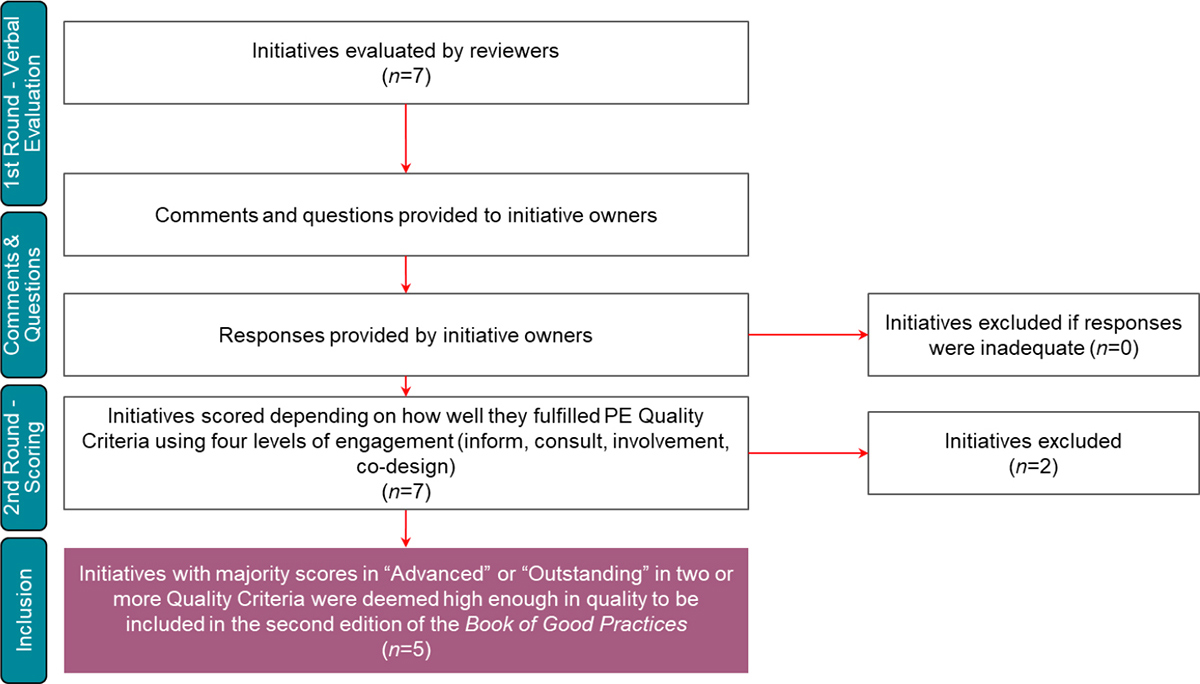
In the second phase, the selected initiatives from the first phase were anonymised and reviewed by the external reviewers in a two-round process. The first round consisted of an overall evaluation of the initiative and how well it addressed or demonstrated each of the seven PE Quality Criteria—with a possibility for reviewers to ask initiative owners for further details in order to obtain a more complete picture of the initiative (Figure 3).
| Level of patient engagement (in Synapse) | Level of exemplification of PE Quality Criteria |
|---|---|
Inform
|
None to low Does not exemplify criteria (Likert: 1) |
Consult
|
Fair/Somewhat Somewhat exemplifies or addresses criteria (Likert: 2) |
Involve (including collaboration)
|
Advanced (Good PE) Good example of the criteria (Likert: 3) |
Co-Design (where higher level is empowerment)
|
Outstanding (Exceptionally PE) Exceptionally high level of PE in the criteria (Likert: 4) |
The five initiatives or “good practices” that passed the review process to be included in the BOGP represent real-life cases where patient engagement has been thought through from the beginning of the project, together with partners. All five initiatives exemplified three or more Quality Criteria, and three of these initiatives exemplified all seven. Although not an exhaustive list, the tables below provide a summary highlighting some of the highly scored Quality Criteria, positive outcomes, and lessons learned by these five initiatives. To explore the five initiatives further, please download the BOGP from PFMD website here.
PE Quality Guidance: A practical tool to facilitate planning, developing, and assessing the quality of PE activities and projects throughout the research, development, and lifecycle of medicines.
Read about the first edition of the Book of Good Practices in the PFMD blog.
Synapse: A patient engagement landscape mapping and networking tool for stakeholders to share knowledge and resources as well as access co-created PE tools.
Conclusion
To summarise the overall reflections of the external reviewers, the BOGP provides stakeholders with a set of graded PE examples, allowing them to compare their practice with others and to identify methods they might use to further enhance their PE. “It not only establishes fundamental criteria for meaningful PE, but also provides ideas and examples of how PE can be done,” and it can be used as a reference tool both by those who are experienced with PE and by those who are just beginning their PE journey.
| Initiative | Pregnancy and Parenting with Arthritis |
|---|---|
| PE Quality Criteria Highlights | 1 – Shared purpose A project proposal was drafted and shared with the [involved stakeholders] engaged in the pregnancy and parenting project. The shared purpose was the subject of regular updates at monthly CAPA* conference calls and email interactions with stakeholders. 5 – Capacity and capability for engagement Ongoing communication with stakeholders was central to the implementation of this project. Because of regular check-ins with stakeholders, there were no issues or concerns with the ability of all participants to contribute effectively and meaningfully. No concerns were expressed by stakeholders at any time during the project. 6 – Documentation and transparency in communication A project page was created on the website to post all relevant project materials for members and other stakeholders. In addition, a resource page was created along with important information highlighting collaborations which can increase patient knowledge relating to pregnancy and parenting with arthritis. Project updates were communicated via the organization’s newsletter and thorough collaboration with arthritis bloggers and other patient groups. |
| Positive Outcome |
|
| Lessons Learned | Given that the project was led by a volunteer patient organization, the project was undertaken over a long period of time. There were difficulties managing resources and time commitments due to the volunteer nature of our work and other life commitments, including managing a serious, complex disease like inflammatory arthritis. |
| Initiative | HeadUp Collar: Co-creation of a new cervical orthosis for patients with Motor Neuron Disease |
|---|---|
| PE Quality Criteria Highlights |
5 – Capacity and capability for engagement
6 – Documentation and transparency in communication |
| Positive Outcome |
|
| Lessons Learned |
|
| Initiative | A sickle cell community built by the community for the community |
|---|---|
| PE Quality Criteria Highlights |
3 – Representativeness of stakeholders
4 – Roles and responsibilities |
| Positive Outcome | The use metrics of the knowledge library identified unmet educational needs of the community, including deeper education about clinical trials and overcoming the barriers to diversity in clinical trial participation. |
| Lessons Learned | A consultation with community leadership at the creation stage of oneSCDvoice.com avoided costly missteps and provided a patient-focused service that truly resonated with the end user as an authentic home on the internet. |
| Initiative | Patient involvement in preparing peer-reviewed clinical research publications or results summaries: A systematic review |
|---|---|
| PE Quality Criteria Highlights |
1 – Shared purpose The shared purpose of this project was agreed upon (1) verbally during author candidate calls and the author kick-off meeting, and (2) in writing with every author signing an official Authorship Agreement that outlined the shared purpose of the project. 2 – Respect and accessibility To help ensure respect and accessibility in this publication project, we:
4 – Roles and responsibilities |
| Positive Outcome |
Positive effects for patients from this project included: Providing patients with access to robust evidence, proving that patients can be involved as authors on peer-reviewed publications. |
| Lessons Learned | Practical tools and plain language documents can and should be prepared to facilitate ethical and effective involvement of patients in publications. Developing these tools and documents takes time, effort, and resources, but they can be re-used and shared to the benefit of others. |
| Initiative | Consulting a patient and career panel on the design and delivery of a proof of concept drug repurposing trial in Parkinson’s Disease |
|---|---|
| PE Quality Criteria Highlights | 1- Shared purpose The overarching purpose of the research interest group was agreed on by members upon joining and by researchers in advance of engaging with the group. The specifics of the activity were agreed on by email and in person during the first meeting chaired by a Parkinson’s UK Public and Patient Involvement coordinator. 3 – Representativeness of stakeholders There was a balanced mixture of representatives in terms of male-to-female ratio, lifestyle, years since diagnosis, and experience with research participation among both patients and family members in the research interest group. This balanced distribution was not specifically selected but arose by chance via the self-selected regular patient group memberships. 6 – Documentation and transparency in communication
|
| Positive Outcome | Co-developing modifications to the study protocol and including the patient voice to documents that were submitted for ethical and regulatory review likely decreased the time to study registration. |
| Lessons Learned | The introduction segment of the session made sure that patient volunteers understood all aspects of the proposed study and felt comfortable asking questions throughout the duration of the project. Their full comprehension was key to co-developing the protocol and emphasized the importance of building relationships with patients to facilitate a frank and open discussion of the ongoing research. Continued involvement will facilitate relationship building and provide an opportunity for patient input at all stages of the research cycle. |

