Identification of Medicinal Products
How to Turn a Compliance Project Into a Strategic Initiative with Additional Benefits
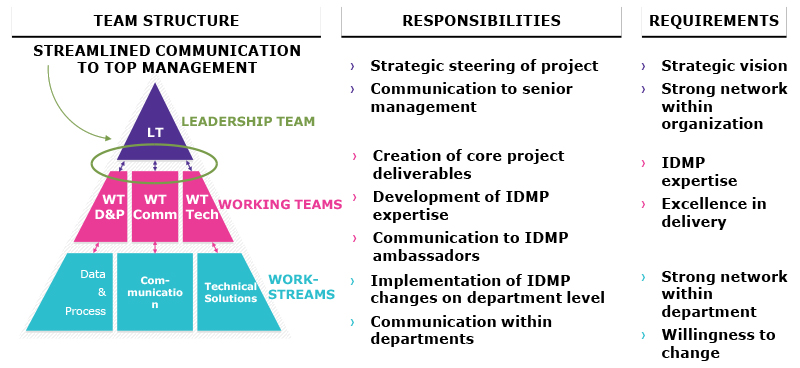
he Identification of Medicinal Products (IDMP) is a master data initiative across the European Union that is based on a set of five international ISO standards. For pharmaceutical companies, a compliance project like IDMP can be turned into a company’s information and data management initiative, resulting in additional benefits on top of being compliant. However, this requires top management support, as well as strong general and change management skills combined with subject matter expertise.
The IDMP concerns the central collection and processing of all relevant information on medicinal products throughout their lifecycle, from development to marketing authorization. Led by EMA, this regulatory compliance initiative is based on a set of five international ISO standards (ISO 11615, ISO 11616, ISO 11240, ISO 11239 and ISO 11238) that define an IDMP data model consisting of data elements and their corresponding relationships for information exchange and the identification of medicinal products. Through the creation of unique identifiers for medicinal products, a track of each pharmaceutical product across its lifecycle leads to a higher level of transparency for patients, pharmaceutical companies, stakeholders, and regulatory activities. The initiative is endorsed globally by other regulatory agencies, such as the US Food and Drug Administration (FDA), Health Canada, and China’s National Medical Products Administration (NMPA).
With a master data approach that includes four core master data domains—SPOR (Substance, Product, Organization, and Referential)—pharmaceutical companies are required to implement IDMP in a phased program based on four defined implementation iterations by EMA. SPOR data management services (SMS, PMS, OMS and RMS) are used to facilitate the implementation of ISO standards. While RMS and OMS went live in 2018 and lay the foundation for SMS and PMS, the latter will be available for product data submission in 2021 (Q3). At the same time, pharmaceutical companies around the EU are working to consolidate and harmonize their SPOR data systems and organizational processes as well as to become compliant with the SPOR-dependent add-ons (such as the electronic Application Form [eAF], Common European [Single] Submission Portal [CE(S)SP], or the Falsified Medicines Directive [FMD]).
For regulatory pharmaceutical processes and data systems, these obligations could have strategic relevance far beyond compliance readiness. IDMP can evolve as part of a company’s information and data management initiative in order to digitize company functions and build advanced analytics within their research and development (R&D) environment and beyond. From a company’s perspective, enhanced operations efficiency, improved decision making, and better patient services are core benefits of IDMP implementation.
Data Management Challenges to Realizing IDMP
Today, business functions and units can act as silos in which cross-functional usage and transfer of IDMP-related data is limited, without any end-to-end view, although some of the same information and data sources are used. Regulators and industry stakeholders require, e.g., referential data (controlled vocabularies, dictionaries, or domain values) as well as terms for regulatory procedures and submissions for clinical trials, marketing authorization applications, or pharmacovigilance across the product lifecycle for products regulated in the EU. Pharma companies must synchronize these referential data with their local vocabulary in systems that are usually administrated and used by other business functions to align internal and external code names and maintain compliance. Because IDMP data rely on many data silos, these data need to be extracted from multiple systems, resulting in huge efforts of interconnecting data silos along the entire medicinal products value chain.
Furthermore, underlying business processes are not always robust enough to maintain compliance in an efficient way. In addition, data mapping and harmonization activities have shown poor data granularity and quality. Correcting and adapting these data to IDMP format on a regular basis can lead to additional costs and a higher probability of an endangered market authorization. IDMP data is often unstructured and in free text form (such as Summary of Product Characteristics [SmPC]), making the manual extraction of IDMP-relevant data elements time-consuming, resource-intensive, and prone to error.
Consequently, data management is challenging not only due to unmanaged data or no appropriate data strategy, but also due to inconsistent reference data and governance across systems as well as a divided responsibility between central authority and decentralized business functions.
Turning the Project Into a Strategic Initiative
Technical Solutions for Data and Processes
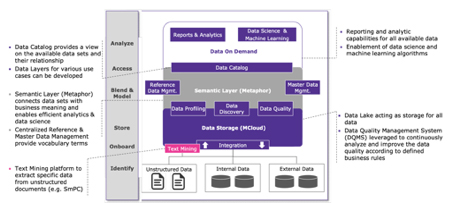
Moreover, data quality is necessary for effective data management. An appropriate Data Quality Management System (DQMS) interconnects various data systems to report data quality according to defined business rules and corrects data according to these sets of rules.
A text mining tool extracts data to be submitted directly from unstructured data (e.g., SmPC) to the right data field from the IDMP data model. For that purpose, text mining needs information extraction, topic detection, tracking, and text summarization as well as text encoding, classification, and clustering functions. Capable of controling internal and external terminologies, Metaphor (Terminology Thesaurus Ontology Platform, Merck KGaA, Darmstadt, Germany) is a key element in the development of these technical solutions. It standardizes terms for data consistency, enables and manages dropdown lists (e.g., of referential data or structured authoring), and thus enriches the overall data search functions across a company.
Figure 1 shows how different data or source systems interconnect with the new technologies and tools for a fully automated submission. This not only achieves compliance, but also establishes one language across the organization, developing a central repository for products, and connecting the clinical sphere to regulatory, or developing one view of the product across the value chain.
Management and Leadership Support
A clear set of goals as well as a supportive management team can strengthen a project team to sustain momentum when unexpected and unpredictable circumstances (such as timeline shifts from external sources) occur.