INNOS
FIFARMA
he adoption of Good Regulatory Practices (GRPs) is fundamental to strengthening countries’ regulatory capacity and ensuring that pharmaceutical product regulation aligns with international standards. The promotion of GRPs by regulatory authorities and other key stakeholders, such as the pharmaceutical and health innovation industry, is essential to improve regulation quality, foster public trust, and facilitate international harmonization and cooperation in every region. But in one specific region, Latin America, the adoption of GRPs is uneven and not universal, thus hampering the alignment of all of Latin America with international standards.
FIFARMA and INNOS launched a study in December 2024 to evaluate the adoption of GRP in pharmaceutical product regulation in eight Latin American countries: Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, Mexico, and Peru. The study is based on data and perceptions collected from professionals working in National Regulatory Authorities for Medicines (NRAs), pharmaceutical companies, and consulting firms that advise the industry on NRA procedures, and brings a comprehensive analysis of regulatory frameworks, institutional capacity, and country-specific challenges related to GRP. This article summarizes the findings of this study.
What are Good Regulatory Practices?
According to the World Health Organization (WHO), more than two billion people lack access to essential pharmaceutical products. This situation particularly affects developing countries, and regulatory barriers are one of the factors limiting the availability of safe and effective treatments. Regulatory systems play a crucial role in ensuring the safety and efficacy of pharmaceutical products, but they are also a vital component of fostering health innovation and improving public health outcomes. Therefore, advancing the maturity of regulatory systems can greatly contribute to tackling those issues.
Good Regulatory Practices represent a set of principles and practices applied to improve the quality of regulation and achievement of expected outcomes. The WHO and the Pan American Health Organization (PAHO) recognize the value of GRPs and invite member states to integrate GRP principles into their regulatory systems and to consider establishing roadmaps, after consultation with stakeholders, to monitor progress in their implementation. The ability to demonstrate consistent adherence to GRP principles is also a key part of the Regulatory Performance Assessment Process (PEP) that WHO uses to define WHO-Listed Authorities (WLA), and is therefore considered a hallmark of any trusted regulator.
First of Its Kind
The report “Assessment of the Adoption of Good Regulatory Practices in Pharmaceutical Product Regulation in Eight Latin American Countries” was an initiative of the Latin American Federation of the Pharmaceutical Industry (FIFARMA), a nongovernmental organization representing the innovative pharmaceutical industry in Latin America and the Caribbean. FIFARMA collaborated with the Instituto de Prospectiva e Innovacion en Salud (INNOS), which carried out the study as part of its activities as a Health Think Tank. Its intention was to generate evidence to enable better processes for connection, development, research, and innovation in the health sector, thus contributing to the evolution and continuous improvement of health systems in Latin America. This is the first study of its kind, and the intent is to collect and publish data every other year, starting in 2024, as part of the regional Observatory of Good Regulatory Practices (GRPO).
GRPO’s mission is to monitor GRP adoption and provide practical tools and relevant data to regulatory authorities and decision-makers in Latin America. It is based on the belief that the recommendations of Annex 11 published by the World Health Organization (WHO) provide clear guidance on how regulatory systems should be structured and operated, emphasizing transparency, independence, and consistency in pharmaceutical product approval processes. By following these recommendations, the observatory aims for countries in the region to adopt a regulatory approach that not only ensures pharmaceutical product quality but also facilitates access to technological innovations, vital for improving health systems.
This first issue of this report (in 2024) evaluates the adoption of Good Regulatory Practices (GRPs) in pharmaceutical product regulation in eight Latin American countries: Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, Mexico, and Peru. The analysis focuses on the aforementioned WHO Annex 11 recommendations, examining regulatory frameworks, institutional capacity, and country-specific challenges. The study aims to identify strengths and opportunities for improvement that contribute to more effective and uniform adoption of GRPs, with the goal of strengthening regulatory systems through efficient processes that ensure timely access and continuity in the supply of therapies for patients.
The information published in this report highlights key areas where regulatory frameworks can be improved, supporting the convergence of regulatory standards with international best practices. This contributes to making National Regulatory Authorities more resilient and capable of responding to emerging health challenges.
Findings and Recommendations
The study methodology included a structured online survey, designed based on the key elements of each principle and enabler established in the WHO reference document, using a rubric (descriptive statement) that aligns the ideal state of each element with WHO standards. The survey rubric included three levels of adoption of principles and enablers—basic, intermediate, and advanced—later transformed into a scoring system that ranged from 1 (no adoption) to 100 (full adoption).
The descriptive data of respondents revealed a varied distribution in terms of experience and areas of specialization. The majority of participants (44%) have 16 or more years of experience in the pharmaceutical sector, suggesting a high level of knowledge. Regarding areas of expertise, Regulatory Affairs dominates with 45.5% of respondents, followed by Pharmacovigilance (19.5%). This combination of extensive experience and specialization in key areas is expected to provide a solid foundation for the assessment of GRP adoption in the region.
Key findings pointed to significant disparities in GRP adoption among the countries studied, with some achieving notable progress, while others face basic challenges in regulatory infrastructure and technical capacity. The findings also indicate an urgent need for greater regulatory harmonization and convergence, and for increased international regulatory cooperation.
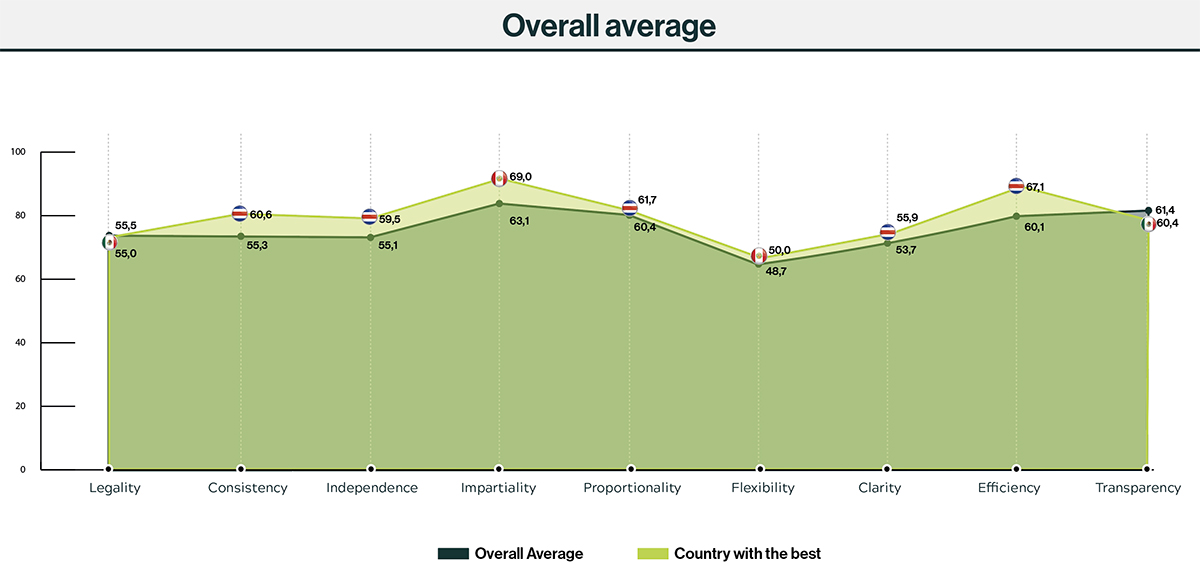
Costa Rica got the best results in five of nine principles, while Mexico and Peru obtained the best result for two principles each.

Figure 2 shows the countries with best results of GRP enablers and LATAM average scores. As well as with the principles, enablers performance is growing in all countries, leaving open space for better results in the future.
Mexico got the best results in three of eight enablers, while Perú and Costa Rica obtained the best results for two enablers each, and Colombia got the best score in one of the enablers.
The study led to the understanding that there is a need to strengthen the commitment of all stakeholders to supporting the adoption of GRPs in Pharmaceutical Product Regulation, as well as their principles and enablers. Moreover, there are opportunities to promote socialization and develop collaborative initiatives focused on the insufficiently implemented aspects of the GRPs for each country, thus facilitating the exchange of progress and experiences. Furthermore, the study indicates that progress is driven by collaborative work between regulatory authorities and other stakeholders, as well as by the adoption of technology. Funding for regulatory authorities remains a critical issue in the region, requiring attention if countries are going to progress in GRP implementation.
Report Recommendations
- Promote the alignment of national regulations with international standards, facilitating coherence and efficiency in regulatory processes at the regional level.
- Adopt advanced technological platforms to streamline the submission, evaluation, and monitoring processes of pharmaceutical products, optimizing efficiency and promoting transparency.
- Establish mechanisms for dialogue and cooperation between regulatory agencies, industry, academia, and patient organizations to address regulatory challenges comprehensively.
- Create and implement standardized metrics (performance indicators) to evaluate the effectiveness of GRP, enabling the identification of areas for improvement and progress monitoring.
- Implement information access policies that allow all stakeholders to understand and participate in regulatory processes.
- Strengthening technical capabilities and regional cooperation.
- Implement artificial intelligence (AI) to optimize regulatory processes and business models in the health sector, leveraging its transformative potential.
- Strengthen the Pan American Network for Drug Regulatory Harmonization (PANDRH).
- Promote expert exchange between NRAs to facilitate knowledge transfer.
- Implement pilot projects for regulatory reliance in pharmaceutical product evaluation and approval processes.
- Establish effective regulatory reliance mechanisms, aligning these mechanisms with WHO recommendations and international best practices.
Moving Forward
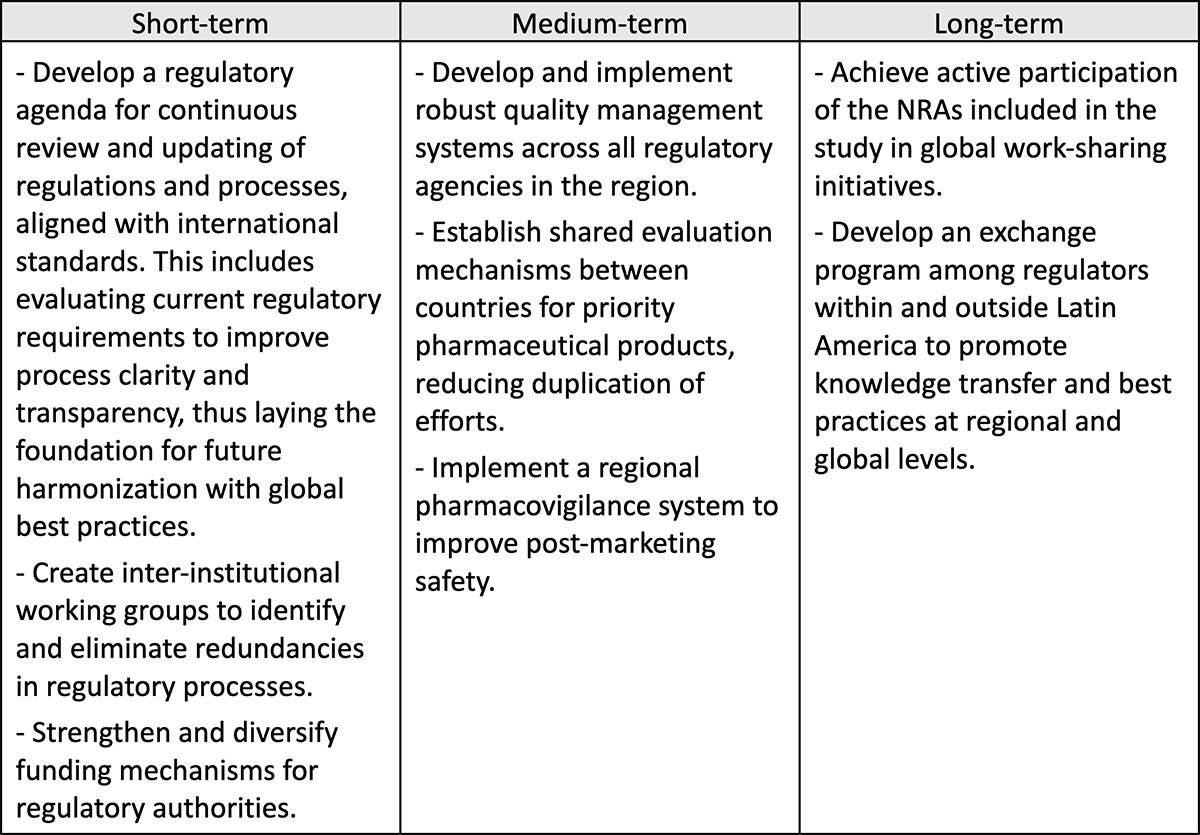
The report suggests potential strategies that can be adopted in the Latin American region in the short (1-2 years), medium (3-5 years), and long (5+ years) terms, so as to improve efficiency and transparency in regulatory systems.
To learn more about this topic, plan to attend DIA’s 2025 Latin America Annual Meeting.

