Olof Lagerlund
Julia Nyman
WHO-UMC
Ta-Jen Chen
US FDA
Isabel Chicharo
EMA
Boehringer Ingelheim
egulatory oversight of medicinal products entails both challenges and opportunities for regulatory authorities, including the fact that today much information about medical products is in electronic form. Electronic information translates into vast quantities of medicinal product and regulatory data, not all of which are uniform in structure, format, and content across jurisdictions. The unambiguous global identification of medicinal products is needed to achieve common international public health goals in areas such as pharmacovigilance, medicinal product track and trace, and to support other international initiatives, such as pharmaceutical quality.
IDMP provides an international framework to uniquely identify and describe medicinal products with consistent documentation and terminologies, as well as to facilitate the exchange of product information between global regulators, manufacturers, suppliers, and distributors. When fully implemented globally, the standards will facilitate the unique identification of medicinal products in the context of pharmacovigilance, drug shortages, and the safety of medications (and patients who take them) throughout the world. How do they do that? By using the unique global Pharmaceutical Product Identification (PhPID).
ISO 11615, Health informatics — Identification of medicinal products — Data elements and structures for the unique identification and exchange of regulated medicinal product information.
ISO 11616, Health informatics — Identification of medicinal products — Data elements and structures for the unique identification and exchange of regulated pharmaceutical product information.
ISO 11238, Health informatics — Identification of medicinal products — Data elements and structures for the unique identification and exchange of regulated information on substances.
ISO 11239, Health informatics — Identification of medicinal products — Data elements and structures for the unique identification and exchange of regulated information on pharmaceutical dose forms, units of presentation, routes of administration and packaging.
ISO 11240, Health informatics — Identification of medicinal products — Data elements and structures for the unique identification and exchange of units of measurement.
These standards for the Identification of Medicinal Products (IDMP) support the activities of medicines regulatory agencies worldwide by jurisdiction. These include a variety of regulatory activities related to development, registration, and lifecycle management of medicinal products, as well as pharmacovigilance and risk management.
In the diagram below, the bottom layer is ISO 11615, known as the unique medicinal product identification standard. This standard describes the data elements and relationships required for the unique identification of medicinal products. Data elements that identify and characterize a medicinal product include the product name (authorized by regulatory agency), clinical particulars (e.g., indications, contraindications), pharmaceutical product (active substance, dosage form, route of administration), medicinal product packaging, marketing authorization (e.g., authorization number, application information), and manufacturer/establishment.
Diagram 1: IDMP Components
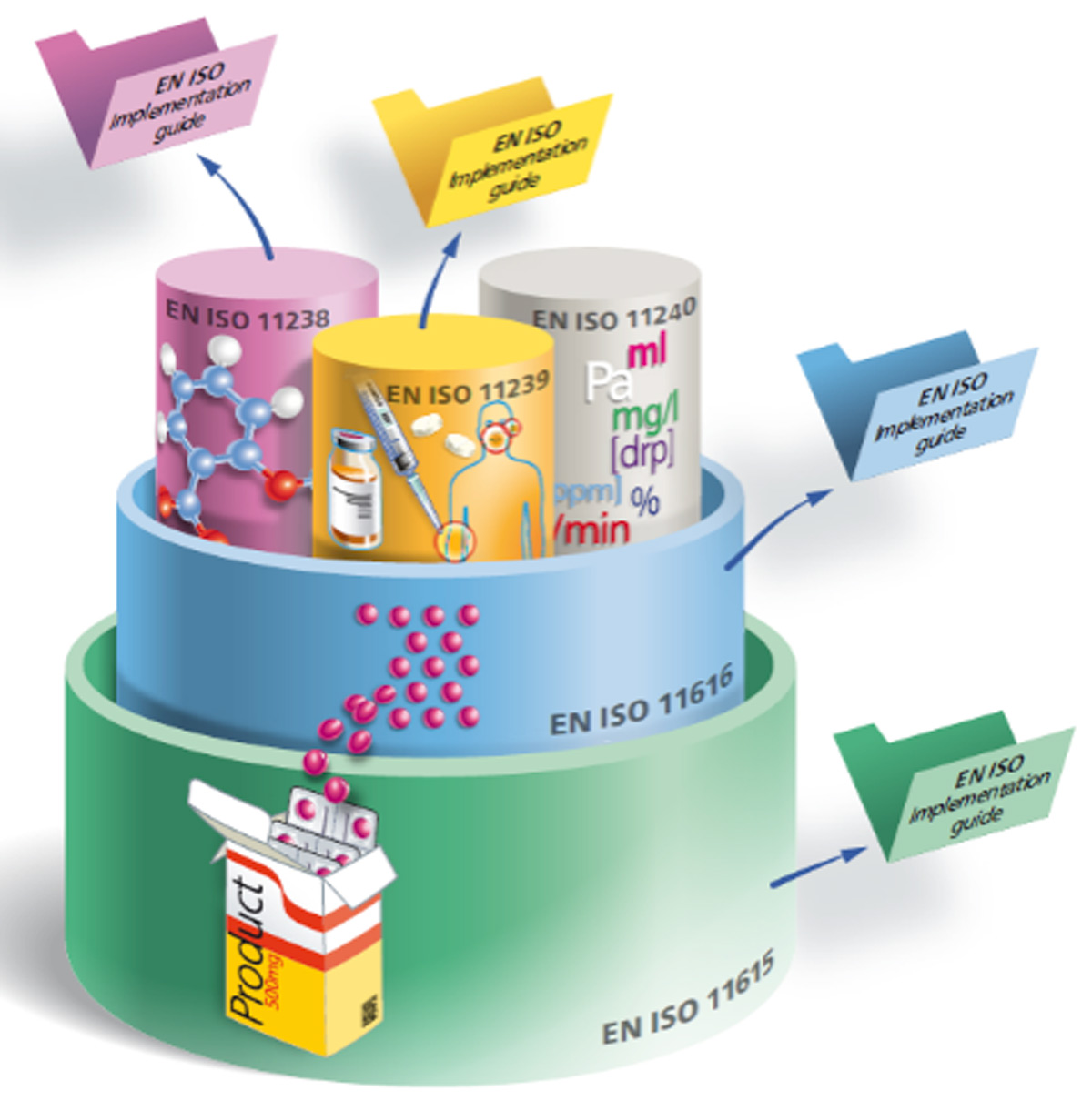
Diagram 1 from ISO/TC 215 Working Group 6.
With its defined data elements and common vocabularies, IDMP will improve data quality and make it easier to harmonize and share information across international regulatory jurisdictions. Improved data quality, and easier and faster sharing of information, will improve our ability to identify, assess, and respond to, for example, a patient’s medication event faster and more precisely. Further, when fully implemented, these standards will make it easier to find alternatives to address medicinal product shortages, and to compare products in one region and across different regions. To maximize the benefits of IDMP standards, global agreement on the substance and dose form identifiers is required.
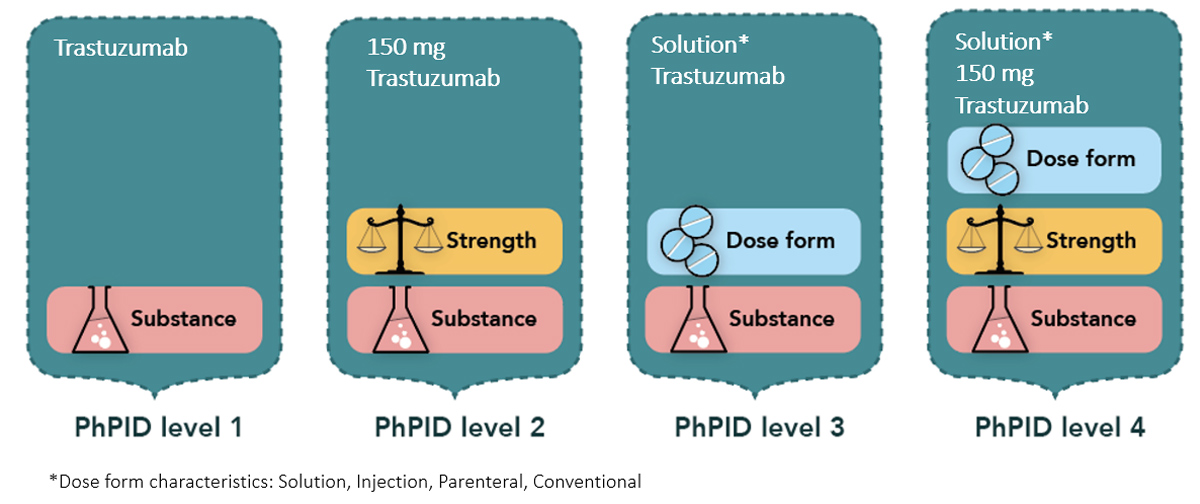
For example, in Figure 1, the PhPID for trastuzumab can be classified at four levels. Level 1 is generated using the substance ID only. Level 2 is substance and strength. Level 3 is substance and dose form. Level 4, the most precise level, includes substance, strength, and dose form information.
So, How Will This Work?
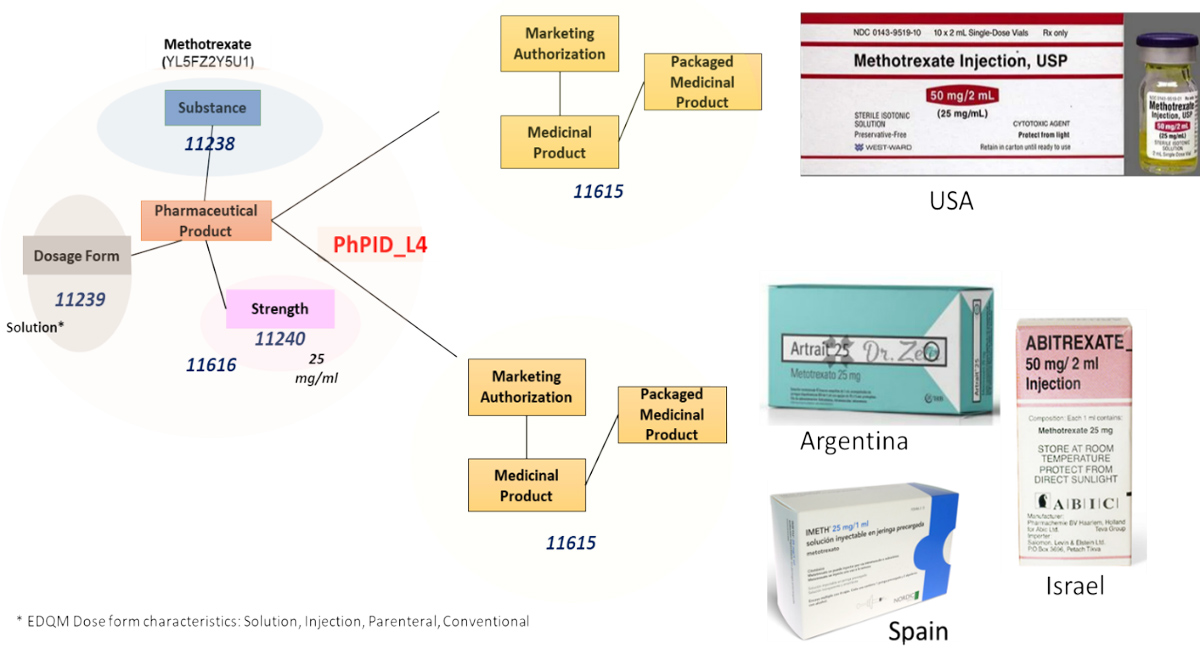
PhPID allows us to connect medicinal products within a region and across regions. Figure 2 shows that methotrexate, 25 mg/ml injection, marketed in the United States is the same as the products in Spain, Argentina, and Israel, because they all share the same substance ID, dose form characteristics, and strength.
Figure 2: How PhPID Works
The implementation and use of ISO IDMP is a regulatory requirement defined in the EU legislation No. 520/2012 (articles 25 and 26), which mandates the use of ISO IDMP for the exchange of information on medicinal products across the European Union (EU). Following the issuance of the legislation, the European Medicines Agency (EMA) issued many guidelines, including Introduction to ISO Identification of Medicinal Products, SPOR programme. In March 2023, the US FDA issued guidance on Identification of Medicinal Products – Implementation and Use, which recommends establishing a framework for the global implementation of the ISO IDMP standards and the maintenance of global identifiers. FDA intends for the framework to ensure that global identifiers are accessible to stakeholders for global pharmacovigilance, supply-chain integrity, and reliable exchange of product information.
Currently, there are challenges with the implementation of the global ISO IDMP standards. The Global IDMP Working Group (GIDWG) has been working with ISO/TC 215 Working Group 6 to address the challenges and to update the standards so that they can be used for global implementation. The following articles provide more information on what the GIDWG has accomplished and its plans for a phased global implementation of the IDMP standards to accomplish the multistakeholder benefits discussed.

