Sonja Brajovic
US FDA
EMA
Marilina Castellano
WHO-UMC
dentification of Medicinal Products (IDMP) standards developed by the International Organization for Standardization (ISO) provides an international framework to uniquely identify and describe medicinal products with consistent documentation and terminologies, as well as to facilitate the exchange of product information between global regulators, manufacturers, suppliers, and distributors. When fully implemented globally, the standards will facilitate the unique identification of medicinal products in the context of pharmacovigilance, drug shortages, and the safety of medications (and patients who take them) throughout the world.
Based on the country reports and further investigation, the World Health Organization (WHO) issued six global medical alerts for substandard (contaminated) pediatric liquid dosage medicines. The investigation identified toxic levels of diethylene glycol and ethylene glycol, known to result in acute renal failure and fatalities. While WHO medical product alerts refer to specific batches of substandard (contaminated) products identified in a specific country, these products may have marketing authorizations in other countries or regions or may have been distributed to other countries through informal markets.
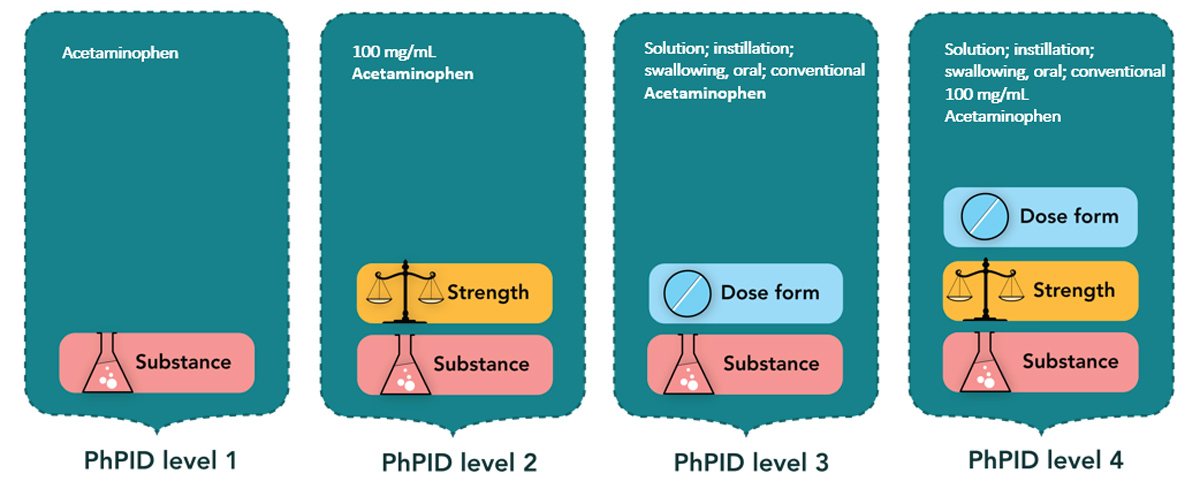
As already mentioned in the first article of the series, the Pharmaceutical Product Identification (PhPID) can be generated at four different levels, depending on the granularity of the information available and based on the standards.
Figure 1: Closer Look at the PhPID
Without a global PhPID, the medical product alert is diffused amongst different product names and their batch numbers. Including global PhPID identifiers in alerts can strengthen regional pharmacovigilance by enabling more effective alert communications. Regulatory authorities across the world could in turn mine their pharmacovigilance databases using Global PhPID level 1, 2, 3, or 4 to retrieve relevant Individual Case Safety Reports (ICSRs), help identify contamination not yet recognized in each given region, and ensure public health safety. Similarly, the alert could be shared with healthcare professionals such as pediatricians and pharmacists, so they can identify over-the-counter (OTC) products for children with the identifiers available in eDispensing and ePrescribing software systems.
In pharmacovigilance adverse event databases, OTC products, when reported, are frequently reported by name only or by the name of the product line. It is likely that the global PhPID level would have been able to aid mining of pharmacovigilance databases to identify similar medicinal products reported in combination with relevant adverse events such as acute kidney injury.
Paracetamol is an ingredient in many OTC products, mainly in tablet/capsule dose forms. If signal detection is at the substance level 1 only, incidents with liquid dose form would be difficult to identify. Global PhPID level 3 would enable identification of all medicinal products that contain the same substance (paracetamol) and the same pharmaceutical form (syrup) and filter out all other dose forms.
In conclusion, more effective alert communication to stakeholders and real-time identification of unexpected serious adverse events in pharmacovigilance databases is possible using global IDMP standards, enhancing public health safety across countries.

