Norman Schmuff
US FDA
EMA
Marilina Castellano
WHO-UMC
he IDMP standards developed by the International Organization for Standardization (ISO) provide an international framework to uniquely identify and describe medicinal products with consistent documentation and terminologies, as well as to facilitate the exchange of product information between global regulators, manufacturers, suppliers, and distributors. When fully implemented globally, the standards will facilitate the unique identification of medicinal products in the context of pharmacovigilance, drug shortages, and the safety of medications (and patients who take them) throughout the world.
Cisplatin is a chemotherapy drug initially approved by FDA in 1978 to treat bladder, ovarian, and testicular cancer, but it is also used to treat a wide range of cancers, including breast, throat, lung, prostate, and colorectal. For many cancer patients, it is the standard of care.
The 2023 cisplatin drug shortage in the US was caused by a quality-related manufacturing issue at one of the primary approved production facilities. This report also indicated that other approved Marketing Authorization Holders were unable to meet the demand for this product. In the US, the marketing authorization holder is required to notify the FDA that the patient demand for a product (in this case cisplatin) cannot be met. Of course, the first step is to reach out to other approved or pending US application holders to see if they can increase production.
Depending on the significance of the shortage, the second step would be to reach out to international regulatory jurisdictions to identify potential non-US sources of the product (e.g., cisplatin). Some of the challenges in resolving a shortage by sourcing in another jurisdiction include 1. Quantity available; 2. May be a different strength and potency; 3. No local or regional distributors (i.e., distribution channels); 4. Time lag to import; 5. Medical product quality verification; 6. Resilience of supply chain; 7. Communication and transparency between manufacturers, regulators, healthcare providers, and patients; 8. Regulatory compliance to ensure patient safety; and 9. Allocation prioritization based on medical needs.
One key aspect of responding to drug shortages is communications to stakeholders through a national drug shortages database as well as sending “Dear Healthcare Professional” letters. The cisplatin shortage was first posted on the FDA drug shortages website on February 10, 2023.
What does this mean for the patient? Drug shortages lead to other consequences, including delays in treatment, dose adjustments, and transitions to alternative therapies. Such alterations raise the spectre of medication errors and adverse events. Newly diagnosed patients will likely not be able to start treatment with cisplatin and will be offered an alternative platinum-based product. Generally, if a patient is receiving treatment with cisplatin prior to the shortage, they would be prioritized over a new patient.
To protect as many patients as possible from being harmed, regulatory action must be prompt. However, identification of a foreign substitute is challenging and time consuming. Initially, the search for a supplier was confined to FDA-sanctioned cisplatin active pharmaceutical ingredient manufacturing sites located in Germany and India. A comprehensive evaluation of global cisplatin facilities proved challenging due to the lack of international interoperability and standardized global identifiers.
In May 2023, four months after the shortage started, FDA announced the temporary importation of non-US-labeled Cisplatin Injection. To swiftly address the shortage, the imported product was not relabeled or repackaged, and this was all explained in a “Dear Healthcare Professional” communication to relevant stakeholders.
But what if we had a global Pharmaceutical Product Identification (PhPID)? What value would it have in remediating drug shortages? A global PhPID would allow the efficient identification of similar products, regardless of where they are approved, for an expedited reach to patients.
Figure 1: Value of Global PhPID to Mitigate Drug Shortages
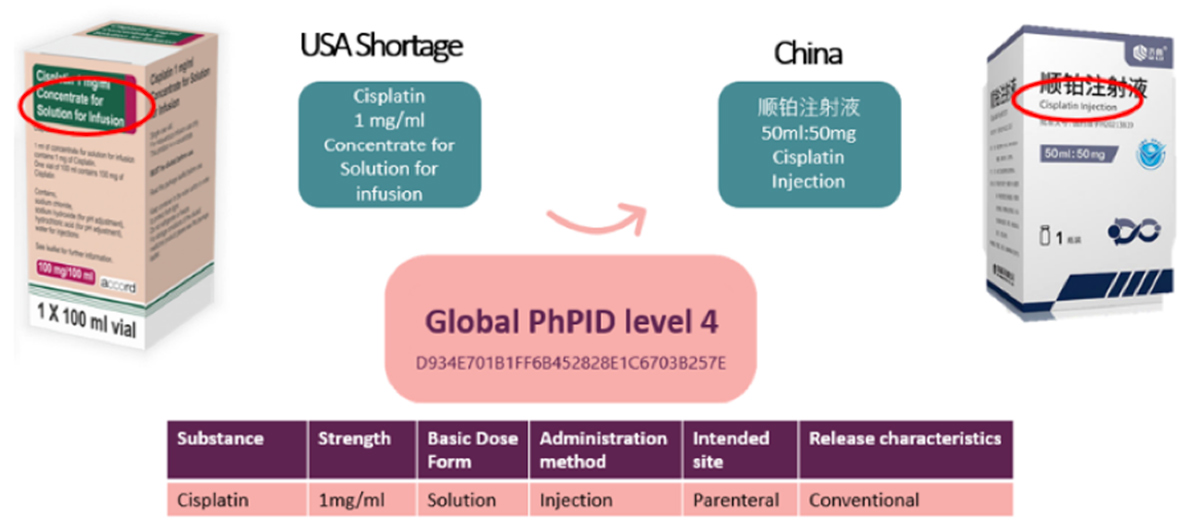
Figure 1 illustrates how two cisplatin products and their pharmaceutical characteristics are described and presented differently. However, with a PhPID, we know the two products share the same global substance ID, the same strength (1mg/ml), and the same dose-form characteristics. When each medicinal product is linked to a global PhPID, it is possible to conclude that these products are the same.
The use of global IDMP standards, and specifically the global PhPID, has the potential to save days to weeks in the initial alternate/substitute drug product identification stage. Quick identification of equivalent medicinal products allows drug-shortage staff to invest their time more efficiently and effectively. In addition, there would be better use of healthcare resources, and, most importantly, prevention of harm to patients by eliminating alternative regimens that may be less effective.

