End in Sight of Transitional Period of New EU Regulations for Medical & In Vitro Diagnostic Devices
Erik Vollebregt
Partner
Axon Lawyers
he new Medical Devices Regulation (MDR) and IVD Regulation (IVDR) entered into force on 25 May 2017, starting a three-year (MDR) and five-year (IVDR) transitional period.
The features of the MDR and IVDR were described in a previous Global Forum article. This is an update about the state of implementation of the regulations.
Transitional Regime
Companies need to prepare to deal with all the changes described in the previous article in a proactive and timely manner to avoid having certificates suspended or revoked because they were unable to comply with the new legislation in time. Fact sheets for specific manufacturers, healthcare institutions and other interested parties have been provided by the Commission in the meantime (see Figure 1).
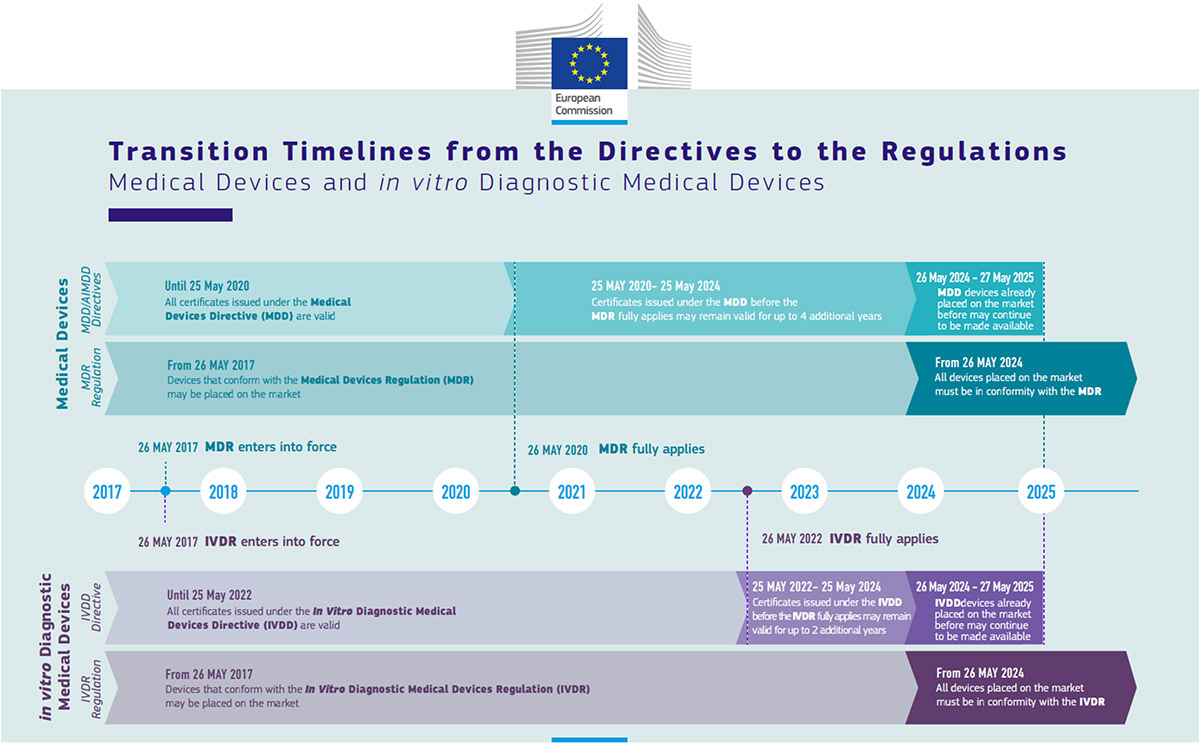
The transitional regime is centered around the transitional period of three years under the MDR and five years under the IVDR. During the transitional period, the notified bodies will still be able to grant certificates under the medical devices directives. However, these certificates are limited in the period of time that they can overrun the end of the transitional period: four years maximum for medical devices and two years for IVDs. In addition, devices on such overrunning certificates may not significantly change any more past the end of the transitional period.
Additionally, devices that are placed on the market in the EU during the transitional period may still be sold for another five years after the expiry of the transitional period.
As a result of the re-notification process for notified bodies, capacity for evaluating medical devices under the new rules is extremely limited and so far only one notified body has been accredited under the new rules. Ironically this is British Standards Institution (BSI UK), which has so far refused to accept conformity assessment applications for the MDR given the uncertainty of Brexit that would interfere with this.
Brexit Bottleneck
Delayed Roll-Out of Implementation Measures
The implementation period for the MDR and IVDR has so far focused on the Commission and member states working on implementing the critical infrastructure for the MDR, which largely (but not completely) overlaps with that for the IVDR. The roll-out plans have been laid down in the CAMD Roadmap and the Rolling Plan. The new EU governing body for the MDR and IVDR, the Medical Device Coordination Group (MDCG), has been slowly adopting an increasing body of guidance, so far mainly on UDI and notified body assessments.
The late adoption of measures necessary for functioning of the new system (e.g., implementation of EUDAMED, the EU database that will contain all devices and economic operators and harmonized standards) made the transition period an uncertain time for industry. Essentially, it means transitioning to a half-finished regulatory system while it is being completed, which means working with many unknown factors in the transition to the new system. This has caused many companies to adopt a “wait and see” approach, which is likely to cause them to run out of time to transition before May 2020. Other companies bet on the corrigendum to the MDR and IVDR to contain an extension of the transition deadlines, and they have lost this bet as the corrigendum did not contain any extension (despite intense lobbying).
The below table shows a very high-level overview of the status of implementation measures and the factors that remain unknown.
- MDR and IVDR text
- Rolling Plan
- CAMD Roadmap
- When to apply at notified body for (AI)MDD recert?
- Corrigendum–dots, commas and no transition amended
- Annex XVI non-medical devices common specifications
- Common specifications (except Annex XVI non-medical devices)
- Implementing acts
- What standards will be harmonised?
- Basically all guidance in CAMD Roadmap (except MDCG stuff on UDI and CAMD Q&A)
- EUDAMED functionality by March 2020
- National implementation measures (enforcement, administrative implantation, etc.)
- When to apply to notified body for IVDD recertification?
- Your notified body ready to accept MDR or IVDR conformity assessment application
- National enforcement in case of
- bottleneck induced shortages
- notified body failing to deliver MDR certificate timely
- Brexit
- MDR and IVDR text
- Rolling Plan
- CAMD Roadmap
- When to apply at notified body for (AI)MDD recert?
- Corrigendum–dots, commas and no transition amended
- Annex XVI non-medical devices common specifications
- Common specifications (except Annex XVI non-medical devices)
- Implementing acts
- What standards will be harmonised?
- Basically all guidance in CAMD Roadmap (except MDCG stuff on UDI and CAMD Q&A)
- EUDAMED functionality by March 2020
- National implementation measures (enforcement, administrative implantation, etc.)
- When to apply to notified body for IVDD recertification?
- Your notified body ready to accept MDR or IVDR conformity assessment application
- National enforcement in case of
- bottleneck induced shortages
- notified body failing to deliver MDR certificate timely
- Brexit
Combination Products
EMA has recently released a new guidance note on combination products in relation to the MDR and IVDR, which is important because one in four centrally authorised medicines in the EU contains a device component. This first guidance note focuses on article 117 Directive 2001/83, as applications for a marketing authorisation (MA) of a medicinal product with an integral medical device submitted as of 26 May 2020 must comply with the requirements of Article 117 MDR. Article 117 MDR does not affect existing MAs for combination products but if there is a substantial change to the design or intended purpose of the device component, or a new device is introduced, after authorization, any required certificate/declaration of conformity/NB opinion should be submitted (as appropriate) to EMA/NCA as part of the variation/extension application.

