Around The Globe
PMDA Puts Regulatory Approval on a Fast Track
Takao Yamori
Executive Director,
Pharmaceuticals and Medical Devices Agency, Japan
Sandra Blumenrath
DIA Science Writer
n recent years, Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) has taken several steps towards innovating the regulatory process to deliver safer and more effective medicines and devices to patients with life-threatening conditions. Restructuring efforts have targeted the very core of the organization, seeking to improve PMDA’s performance efficiency throughout the regulatory review process.
To that end, PMDA has implemented various innovative, fast-track regulatory initiatives as part of its current 5-year Mid-Term Plan, including the sakigake Designation System and the most recent Conditional Early Approval System.
The result: PMDA scored a major success with the speedy approval of a “titanium bridge” (NPC-17) for type II thyroplasty – a device whose approval would have remained in limbo considerably longer without the sakigake fast-track review system. With the recent regulatory initiatives, PMDA has met the major targets of its Mid-Term Plan, achieving shorter approval times, greater review service quality, and enhanced safety measures. By prioritizing innovative products and companies that have not filed for approval elsewhere, the ministry of health hopes to provide such products earlier to patients.
What’s New? Conditional Early Approval System
Put into practice in 2017, the Conditional Early Approval System allows for early approval of highly needed, innovative medical devices and speeds up the practical application of drugs that target serious, debilitating diseases. PMDA initially enacted the conditional approval pathway for medical devices (July 2017) but has since followed up with an expansion for pharmaceuticals (October 2017). As with other conditional pathways, this approval system admits early applications without confirmatory clinical trials, on the condition that the product’s safety and efficacy be further evaluated while on the market; what’s more, products eligible for Conditional Early Approval are not restricted to orphan drugs.
To be eligible, pharmaceuticals have to meet four criteria, two of which are already included in conventional priority review:
Same as priority review:
1. Seriousness of indication
- Disease has significant impact on lives
- Disease is progressive and irreversible
2. Medical usefulness
- No existing remedies
- Greater efficacy and safety, and fewer physical and mental side effects than current treatments
Additional criteria:
3. Confirmatory clinical trials are difficult to conduct or take a long time due to a limited number of patients
4. Clinical trials other than confirmatory trials have shown a certain degree of efficacy and safety
With this system, PMDA hopes to accelerate and provide an increased incentive for the development of treatments targeting progressive, life-threatening diseases that are currently incurable.
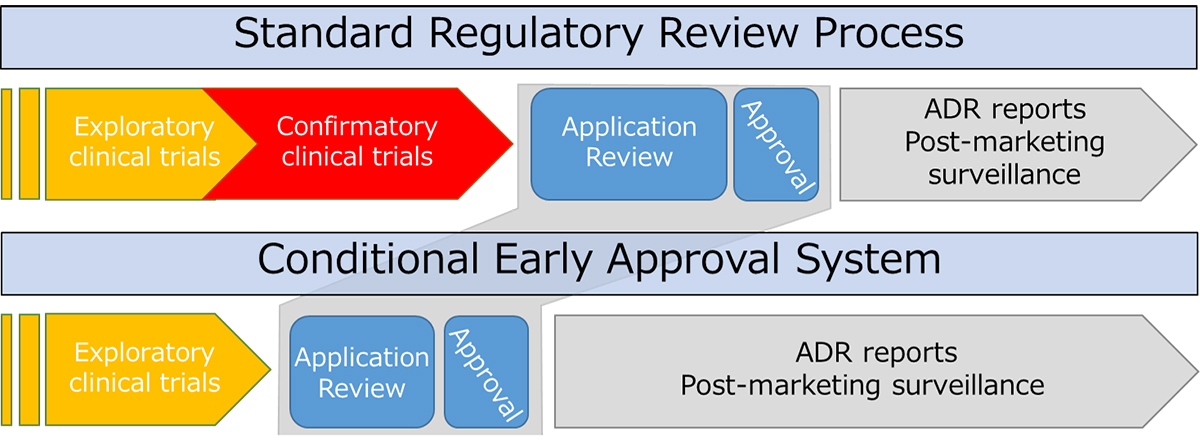
Compared to standard review, Conditional Early Approval significantly speeds up the approval process, bringing life-saving treatments to patients faster. Safety and efficacy are further monitored in the much longer post-market phase.
Speedy Approval of First Sakigake-Designated Device
On December 15, 2017, the Japanese health ministry approved Nobelpharma’s titanium bridge (NPC-17) medical device, the first product to earn approval among all drugs and devices currently designated under the sakigake fast-track pathway.
Implemented in 2015, the sakigake (or “Forerunner”) system allows for certain innovative drugs, medical devices, regenerative products, and in vitro diagnostics to progress more quickly from bench to bedside by working more closely with sponsors during development and by cutting review times in half (from 12 to 6 months) . It offers ongoing regulatory support and data review throughout development and requires stronger post-marketing safety measures in exchange for faster approvals.
The titanium bridge, which treats adductor spasmodic dysphonia (AdSD, a voice disorder), was the first medical device to receive the sakigake designation and emerges as a success story from the first round of reviews that started in 2016. Filed for market approval in July 2017, the device was approved less than 6 months later, in December 2017. Titanium bridges have significantly improved the quality of life for patient with AdSD.

