Scientific and Regulatory Perspectives
ene therapy medicinal products (GTMPs), which in the European Union (EU) are a subset of advanced therapy medicinal products (ATMPs), may have the potential to transform the treatment landscape for many diseases ranging from inborn errors of metabolism to malignancies, offering the promise of treating diseases that may range from debilitating to fatal. The science of gene therapy (GT) was catalyzed by breakthroughs in DNA technology that led to research strategies aimed at replacing or adding genes. These techniques often employ viral vectors.
Gene Therapy: Definitions and Regulatory Challenges
Despite the promise of GT, the regulatory framework is fraught with complexity. A major impediment is the absence of global harmonization of regulatory requirements and definitions. GT is subject to disparate classifications worldwide. In the EU, under current legislation GTMP is precisely delineated as the application of recombinant nucleic acid for the purpose of regulation, repair, replacement, addition, or deletion of a genetic sequence. In contrast, the US FDA offers a broader categorization of GT products, encompassing all products that mediate their effects through the transcription or translation of transferred genetic material or by specifically modifying host (human) genetic sequences. In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) groups GTMPs under the more inclusive label of “regenerative medicine product,” which includes products “containing genes to be expressed.” Moreover, current definitions often do not directly address novel technologies, leading to ambiguous interpretations and classifications even as innovation progresses.
In April 2023, the European Commission (EC) proposed a new pharmaceutical legislation safeguarding public health within the EU. A pivotal amendment under Article 4 (point 29) of the new directive involves redefining the term GTMP to incorporate genome editing techniques and synthetic nucleic acids, which were previously categorized under chemical medicinal products (MPs).
- a substance or a combination of substances intended to edit the host genome in a sequence-specific manner or that contains or consists of cells subjected to such modification; or
- a recombinant or synthetic nucleic acid used in or administered to human beings with a view to regulating, replacing, or adding a genetic sequence that mediates its effect by transcription or translation of the transferred genetic materials or that contains or consists of cells subjected to these modifications.
Mechanism of Action: Scientific and Regulatory Implications
With the recent worldwide approvals of the first mRNA-based vaccines (elasomeran and tozinameran) and CRISPR/Cas9-based therapy (currently approved in Bahrain, the EU, the UK, the US, and Kingdom of Saudi Arabia), we believe it is important to consider the MoA of these groundbreaking products for the purpose of accurate regulatory categorization.
The use of mRNA is based upon a tenet of molecular biology, i.e., that mRNA encodes proteins required for the organism’s function/survival. mRNA therapeutics are based upon synthesizing mRNA that encodes proteins that may be missing/defective. Rather than inserting a functioning gene into the human genome to transcribe mRNA that will then be translated to synthesize proteins on a continuous basis, this approach provides direct translation from mRNA to protein for as long as the mRNA molecules are not yet degraded by natural mechanisms. Importantly, these mRNA therapeutics do not integrate into the host genome and are eventually degraded.
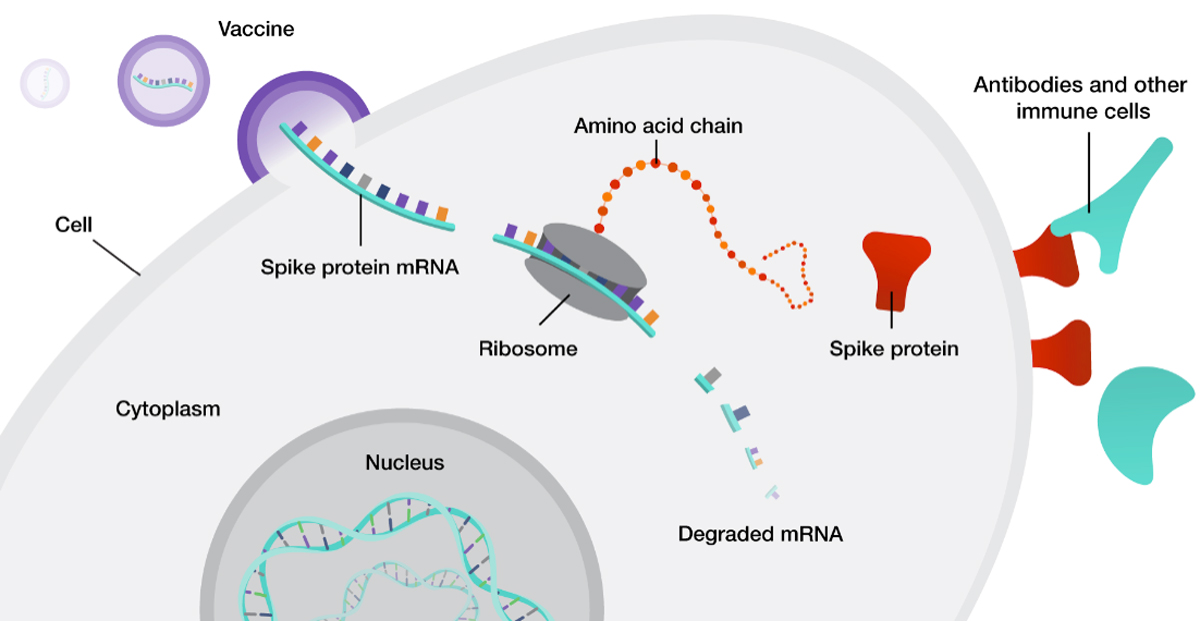
In European legislation, vaccines for infectious diseases are excluded from the GTMP category. However, mRNA-based MPs (Medicinal Products) for other indications including therapeutic vaccines (e.g., in cancer) would fall under the GTMP classification also under the new proposal. The definition of GTMP is grounded in public health considerations, as the persistence and genomic integration of gene-targeting technologies vary widely, from genome editing to transient expression. Expanding understanding of gene-targeting mechanisms and the introduction of new methodologies present an opportunity to refine existing legislation to reflect the current state of knowledge.
Benefit-Risk Assessments in the Development of Gene Therapy Medicinal Products
The advancement of all new MPs, including GTMPs, is predicated on a rigorous benefit-risk assessment. For interventions involving genome editing or genomic integration technologies, a comprehensive evaluation of off-target effects and integration patterns is essential to substantiate the therapeutic benefit and associated risks. Genome editing or genomic integration have potential for certain risks such as insertional mutagenesis, or gene mutations due to off-target effects. Insertional mutagenesis may activate oncogenes or deactivate tumor suppressor genes, raising the risk of oncogenesis. These kinds of risks directly related to the MoA of the therapy are well monitored and controlled during pharmaceutical development. Based on the generated safety and efficacy data, the regulators make conclusions about the benefit-risk ratio at multiple stages during development as well as at registration. Importantly, mRNA-based MPs rely on a different MoA which does not involve genome alterations. Recognizing distinct technologies and their associated characteristics is essential for the benefit-risk assessment that is incorporated into the appropriate regulation of each approach.
Demystifying Gene Therapy for Public Understanding
The term “gene therapy” is widely used to denote gene modification, insertion, or correction. The public is increasingly aware of existing technologies as toplines in the press but is generally unaware of the different MoAs used in GT or of the potential implications of the differences. The lack of understanding may lead to misunderstanding and miscommunication since it does not involve risk and benefit considerations.
This disconnect became apparent during the COVID-19 pandemic, when unfounded concerns about genetic modification contributed to vaccine hesitancy, even though mRNA vaccines do not modify the recipient’s DNA. Such spurious concerns have been linked to reduced vaccination rates and increased mortality. There is a certain risk that such misperceptions may also impact the acceptance of mRNA-based therapeutics as potentially transformative treatments, potentially leading to lower adherence to treatment regimens and premature cessation of therapy.
While we cannot expect lay members of the public and patients to understand the precise MoA and related risks, or lack thereof, for novel therapeutics based on interventions involving “genes,” a regulatory definition can provide this understanding. Future iterations of the GTMP definition could carefully address this by differentiating therapeutics based on their MoA and the related potential risk to patients. Lack of differentiation with regard to risk may confuse the medical and general community, impacting the perception and acceptance of potentially important new therapeutics to address unmet medical needs. More precise definitions and classifications, including what should or should not be included in the term GTMP, may help to avoid misconceptions, enhance acceptance, and encourage the use of potentially transformative therapies, thus contributing to public health. It would also serve the principle of proportionality that is a major driver of the legislative proposal. However, it is not yet known when the legislative proposal, whether in its current form or after any modifications, will be approved.
Conclusions
The ongoing revision of pharmaceutical legislation in Europe presents an opportunity to reevaluate the GTMP classification, adapting it to consider exciting new technologies. It will be important to carefully assess the impact of any definition on patient access and adopt a contemporary and precise definition that reflects each technology’s MoA and risk profile. This approach could support a sound understanding of these innovative therapies so that their benefits and risks are accurately understood. We look forward to the European Commission’s forthcoming deliberations on whether treatments that do not lead to alterations in the human genome, such as mRNA-based therapeutics, should be categorized within the GTMP definition.

