he shift to hybrid, decentralized trials has highlighted the need for better data management and direct digital connections that link sponsors, sites, and patients.
“Before the pandemic, every company had started to dip its toe into the digital world, but COVID-19 pushed us all into the pool. We have had to come up with solutions really quickly to keep our studies afloat, and now it’s forcing us to be a little bit more forward-thinking,” said Lorena Gomez, senior director, global study start up, PRO management, and digital implementation at AbbVie.
To improve clinical trial efficiency and quality, the industry must shift to a complete and connected digital trial ecosystem that allows for seamless data exchange and execution. Clinical leaders will need to rethink trial strategies, move beyond decentralization, reduce the technology burden placed on sites and patients, and establish a clinical database foundation to make this a reality.
Veeva’s Digital Clinical Trials Survey, which surveyed more than 280 clinical leaders from sponsors and CROs, found that more companies are working with decentralized trials, with 87 percent currently using them and 95 percent expecting to increase use over the next year.
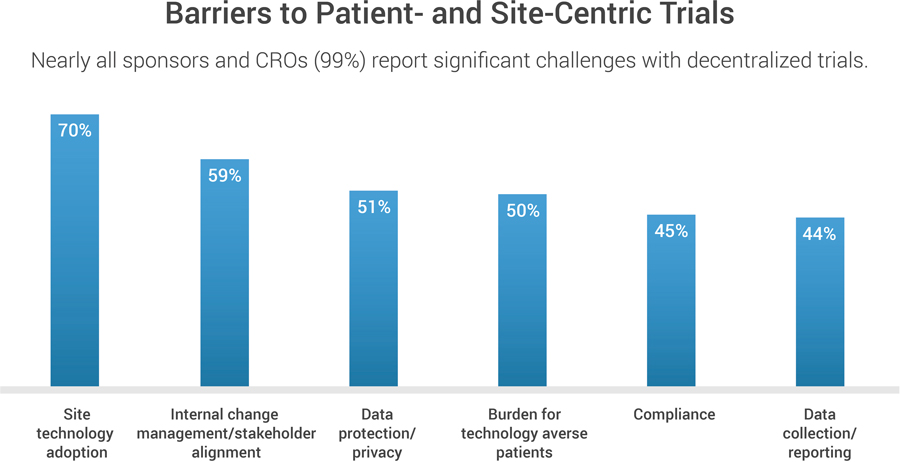
However, many of the early COVID-19 decentralized trials underscored inadequacies. Lingering manual and paper-based processes were found, as well as disconnected eClinical solutions at the patient, sponsor, CRO, and research-site levels. “It’s great to have digital, but now investigators have 10 logins instead of one. It’s challenging from a site perspective to be able to execute trial processes through 10 different systems,” says Staci McDonald, executive director of scientific clinical operations at the research site network Celerion.
As industry considers the next step in clinical operations and data management, the end goals haven’t changed. Sponsors still want trials to run as efficiently as possible; patients need easier ways to connect with sponsors and sites; and sites want to simplify operations so they can focus on patients. But now, industry must evolve beyond a “one-size-fits-all” thinking about decentralization into a deeper understanding of site- and patient-centricity, and a connected approach that will simplify data sharing and enable seamless execution.
To reap the benefits of decentralization, trials must become wholly digital from start to finish, with technology optimized for site and patient convenience, which will:
- Promote easy, effective patient engagement.
- Bring remote capabilities to the research site.
- Accelerate the move to site-focused, -owned, and -controlled site operations systems.
- Extend data cleaning, aggregation, and management from sponsor “back-end” systems into better connection with patient-facing “front-end” clinical operations.
“It’s important that we collaborate strategically, as an industry, to understand how decentralized trials will affect the patient journey and site relationship,” says Mark Morais, president of clinical operations and commercial solutions at LabCorp Drug Development.
Sponsors and CROs see a need for simplification and standardization, and a greater focus on data. “We have to think about the impact to sites, protocol design, and patients, especially in terms of what we’re capturing and how. We must let digital drive simplicity as we potentially increase all the disparate data sources,” Morais says.
Decentralized trials will, by definition, require much more data—most of it from wearables and sensors—and that data will need to be ingested, aggregated, and cleaned, says Mayank Anand, vice president and global head of data strategy and management at GSK. “The sharing of data itself is a challenge. Every study brings its own complexity because you have 10 to 15 different external data providers, and everyone uses a different way to inject data. How will we standardize once we go to 20 or 30 different data sources? We need to bring simplicity and a connected architecture,” he suggests.
To enable this simplicity, sponsors, CROs, and sites will start to take a unified digital approach to clinical trial data and documentation: an approach that standardizes processes and speeds access to relevant data, whether for study planning and setup, site activation, monitoring and management, document and data management, or study closeout.
Vital Role for Research Sites
Digitizing information will improve its flow. We estimate that it can reduce trial costs and timelines by 25 percent and lead to more effective communication and data and document sharing in a decentralized trial environment. This strategy demands a deeper understanding of patient and site preferences and needs, with a more robust data standardization and management approach.
Patient Convenience
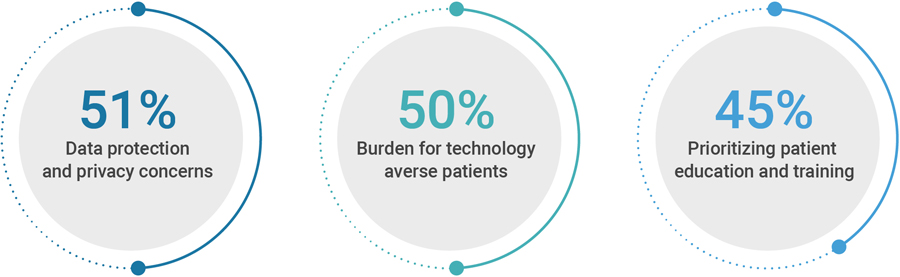
Eliminating the technology burden for patients is another priority. In exchange for reducing the need for travel to sites, the first generation of remote COVID-19-era trials often weighed patients down with numerous digital applications, each designed for one specific function (e.g., registration, site communication, or dosage compliance). Sponsors and CROs recognize the need to address this problem.
Experience with recent decentralized trials also suggests that approaches to patient engagement will need to be varied and personalized. The 19-year-old participating in a trial of a new acne treatment and the 80-year-old cancer patient taking part in oncology drug research will have different preferences. Even the same patient may prefer different interactions at different times during the same trial.
Source: Veeva Systems
In addition, human contact with healthcare providers will remain crucial throughout each study, ensuring a key role for sites well into the future. Study designs will continue to incorporate multiple modalities—both digital and human—for patient care, ensuring patient-centricity and compliance.
Closer Sponsor-Site Collaboration
Source: Veeva Systems
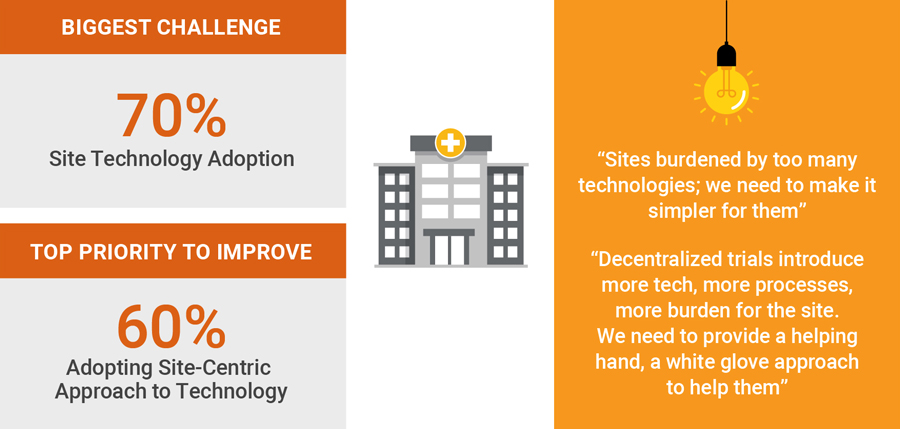
One key to driving better sponsor-site partnerships is empowering sites with technology that meets their specific needs and makes it easy to share information. Industry has a long way to go in this area, but progress is being made: 60 percent of the CROs and sponsors say they are taking tangible steps to reduce site burden by improving data sharing and systems interoperability.
Speed Bumps and Rising Expectations
Source: Veeva Systems
Laying the Foundations for Change
Data: The Final Frontier
As modern digital clinical trial designs evolve, the need to collect, ingest, aggregate, and clean higher volumes of disparate data from many more sources, including patient-facing apps and medical monitoring devices, will be urgent. Currently, this work must be done manually by teams of experts, requiring significant time and resources that would be impractical, if not impossible, to devote to each trial within each company’s clinical portfolio.
Clean and standardized data will be crucial to making clinical research more agile and enabling more advanced approaches such as artificial intelligence in the future. Several top 20 pharmaceutical companies are working on clinical databases that would speed data cleaning and standardization to allow the data to be managed, reported, and analyzed more quickly.
Although challenges remain, industry is applying advanced approaches to manage and share clinical data and documentation as it redefines clinical trials and brings them into the digital age. Sponsors are also developing a better understanding of patient and research site needs and strengthening connections with both groups, resulting in a more sophisticated approach to patient-centricity.
The goal for digital trials is a holistic platform that connects sponsors, sites, and patients for seamless execution and data flow to meet their diverse needs. Currently a work in progress, this platform would reduce trial costs and timelines by 25 percent. As it moves from concept to reality, clinical operations promise to become more innovative and better serve patients by speeding their access to medical breakthroughs.

