Around the Globe
he Latin America region offers a number of registration pathways to accelerate the regulatory assessment of innovative medicines. As the authors of the recent publication Registration pathways to accelerate regulatory assessment of innovative medicines in Latin America in the Journal of Public Health Policy (JPHP), we conducted and published an extensive review of regulatory information collected from 2017 to 2019 for nineteen Latin America (Latam) countries by characterizing the regional regulatory scenario related to accelerated registration pathways and identifying similar opportunities for countries in Latin America. Those findings published in JPHP are summarized here.
What Are Accelerated Regulatory Pathways?
There is extensive information available in the public domain on accelerated pathways in Europe, in the US and in other major countries, but not in Latin America. The JPHP publication intends to cover this gap, with the aim of helping regulators, industry professionals, patient associations and the public gain access to information of high public health relevance. The article also aims to provide, summarize and update information (in English) on the mechanisms used in Latin America, to reach out to those outside the region with limited or no skills in the local languages (Spanish and Portuguese) or other regional expertise. The consolidated data it provides may contribute to the appropriate use and improvement of these accelerated regulatory pathways in the region.
Key Findings: Accelerated Regulatory Pathways in Latin America
In this context, the definition of “reliance pathway” is in line with the concept from the World Health Organization (WHO), also used by the Pan American Health Organization (PAHO). It states that reliance occurs when “The National Regulatory Agency (NRA) in one jurisdiction may give significant weight to–or rely upon–evaluation by another NRA or trusted institution as the basis for reaching its own decision. The relying authority remains responsible and accountable for decisions taken, even when it relies on the decisions and information of others.”
The definition of expedited review was adapted from a White Paper published in 2017 by the European Federation of Pharmaceutical Industries and Associations (EFPIA): “Regulatory authorities shorten duration of product reviews to enable faster approval, most commonly applying criteria for assessing potential benefit of the product for the health system.”
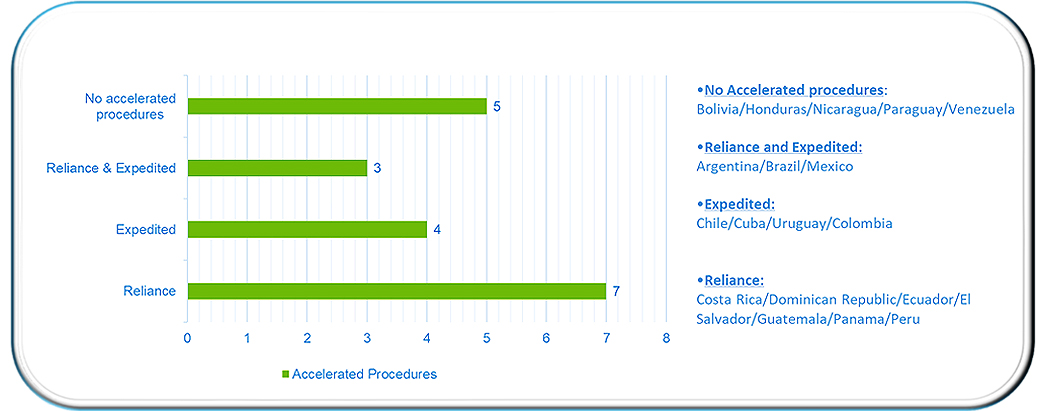
- Need to submit documentation of the approval (known as “CPP: Certificate of Pharmaceutical Product”) of a reference country and, if required, at which stage to submit it (at initial submission of the Marketing Authorization Application [MAA] or during the review of the MAA).
- If CPP is required at initial submission, the sponsor can only submit the MAA after approval in a Reference Country, which will delay your submission significantly.
- The second option allows the sponsor to submit the CPP during the review, so the authority can start to review the MAA before any Reference Country Approval.
- In Latin America, the authors recommend CPP submission during the review, to avoid submission delays, because the CPP does not replace full regulatory review.
- Acceptance of regulatory data for approval purposes based on phase 2 studies, when applicable.
- Registration timelines for accelerated versus standard pathways, based on the authors’ experience and/or extracted from regulations.
- Author experiences with using accelerated pathways in submissions to regulators in Latin America.
Conclusions and Opportunities
The JPHP publication illustrated that the great majority (14 out of 19) of countries in Latin America have established at least one regulatory mechanism to reduce review timelines. Although the authors’ experiences are limited–for example, some of these accelerated pathways were just recently adopted relative to the time of data collection–it is already possible to observe that the use of accelerated registration pathways has reduced overall regulatory review timelines in most cases. In conclusion, with most of the countries studied having at least one accelerated regulatory mechanism in place, the overall regional profile seems positive.
But more opportunities for the region still exist:
- Adoption of accelerated pathways in countries where there are none.
- Accepting CPP, when required, during regulatory review and not at the time of initial submission (see above).
- Reducing of the need for two assessment reports (US FDA and EU EMA) for Regulatory Reliance purposes to only one (this alone may be enough to make accelerated pathway timelines more attractive).
- When justified by benefit-risk, accepting phase 2 studies for approval (which can also considerably expedite approval).
- Adopting WHO Good Regulatory Practices to improve regulatory efficiencies.
Acknowledgment: The authors of the JPHP article are Ana Padua, Livia Partika, Denise Bonamici, Juliana Rahal Cabello, Cristiane Kohiyama, Paula Spinardi, Anabelle Castro, Alessandra Rolim and Francisco Souto. The authors acknowledge Rosana Mastellaro (SINDUSFARMA) for assistance in writing this manuscript.
Note: This publication covered the regulatory scenario from March 2017 to November 2019. Regulatory updates took place after this timeframe in Brazil, Chile, Colombia, and the Dominican Republic.
Disclaimer: The views and opinions expressed in this article do not necessarily reflect the official position of the pharmaceutical industry companies with which the authors were or are currently affiliated.

