Tufts CSDD
Parexel
linical investigative sites face unprecedented operating challenges, from increasing protocol complexity to resource constraints, protracted budget negotiations, and workforce turnover, to contending with myriad new technologies. At the same time, sponsors and CROs (Clinical Research Organizations) attempt to determine how best to collaborate with the sites and aim to become a top “sponsor/CRO-of-choice” in their quest to improve site performance. Inspired by the findings of a survey the Tufts Center for the Study of Drug Development (CSDD) conducted in 2022, as well as their own mission to improve site partnering, a global CRO was interested in further exploring opportunities to obtain a deeper understanding of root causes related to the complexities of sponsor/CRO/site relationships and address site operating challenges.
Research Methodology
A comprehensive literature search was conducted by the Tufts CSDD to identify the primary themes and “pain points” or challenges that sites contend with on a regular basis. The review included articles related to Sponsor/CRO/Site Relationships, Site Engagement, Site Burden, and Sponsor/CRO of Choice from 2011 to 2023.
These pain points were then categorized by relative priority, based on how often the topics were mentioned in the literature (Table 1). The priorities were ranked in terms of level of urgency with which sites believed sponsors and CROs need to address the issues in order to:
- Minimize site burden
- Ensure site sustainability
- Secure their position as a sponsor/CRO of choice for sites.
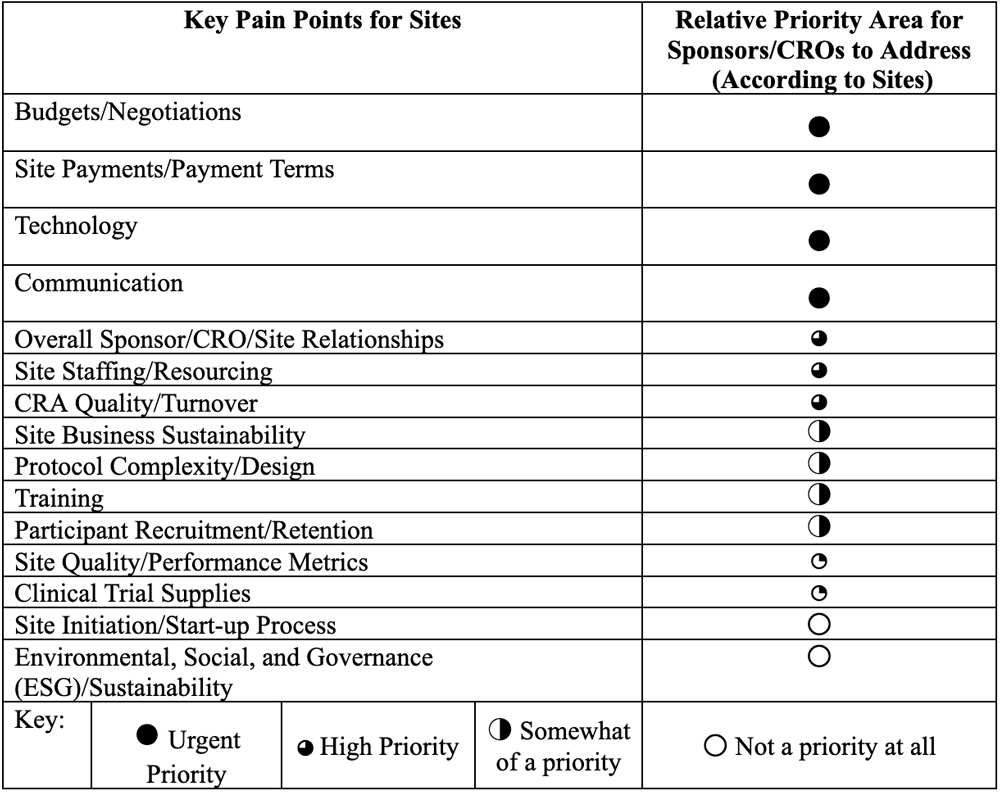
This list helped to inform the areas for in-depth exploration during a series of site interviews. The focus of these interviews was to:
- Determine underlying drivers and causes of these challenges and pain points.
- Identify pragmatic and actionable practices and solutions to optimize the quality and effectiveness of sponsor/CRO-Site relationships.
The CRO identified representatives from different site types and different roles to participate in the interviews, which were conducted by Tufts CSDD staff. A total of 22 interviews were completed across a mix of clinical and administrative site personnel from sites working in different types of clinical research settings, including academic medical centers and hospitals, researchers in community-based health systems and site networks, and dedicated research sites. While some challenges sites face are shared globally, this research focused solely on sites in the US.
Site representatives were first asked to identify their top three pain points from the list of areas identified in Table 1, which guided the flow of the one-hour interviews. Where time allowed, however, all areas were explored. Site representatives were asked to focus their insights and recommendations on the most actionable areas where sponsors and CROs could improve their site support and site relationships. To encourage candor in the responses, interviewees were informed that none of their comments would be attributed to them when the findings were shared and published.
Key Findings – Pain Points and Root Causes
In-depth interviews validated the themes that emerged from the literature review and confirmed the root causes associated with many of these themes. No notable differences were observed in terms of challenges and opportunities between clinical or administrative site personnel across different site types. While several sponsors and CROs are partnering well with sites in some areas, the interviews did uncover substantial opportunities for improvement, many of which could be immediately implemented.
Key themes were:
- One-Size-Fits-All relationships/failure to “customize” based on site type, needs, and experience
- Poor communication flow and lack of responsiveness
- Nonintegrated (or disconnected) technology usage and inadequate support from sponsors/CROs for sites
- Insufficient budgets and negotiations by sponsor/CRO staff who are unfamiliar with the study and/or site operations
- Low CRA quality and high CRA turnover
- Cumbersome feasibility and start-up processes.
More detailed feedback around these themes provides additional context to illustrate the nature and degree of sentiments and frustrations for each pain point. Verbatim quotes are noted below as examples encapsulating the feedback from the collective survey respondents.
One-Size-Fits-All relationships/failure to “customize” based on site type, needs, and experience
Interviewees expressed frustration with what they collectively referred to as a “one-size-fits-all” mentality with which sponsors and CROs approach and interact with them. For example, different site types have different operating models, resources, and other unique attributes that often go unrecognized by sponsors/CROs. Some sites have a full infrastructure in place with a large staff that aligns with multiple stakeholders at sponsors/CROs, but other sites have a small number of core staff, each covering several roles/activities and working with many different staff at the sponsor/CRO. The extra burden is on the latter sites to adapt and accommodate to the sponsor/CRO processes rather than tailoring processes to different types of sites. The lack of dedicated staff at the sponsor/CRO to really learn and understand the sites, coupled with staff turnover on the sponsor/CRO side, require sites to continuously reinvent and reinvest in relationship building. On top of that, because of staff turnover, any sponsor/CRO institutional knowledge about the site is lost, which results in inefficiencies on the part of the site that must continuously re-educate the sponsor/CRO about the site (or site network) and its operations, systems, and processes.
Sites are further exasperated by what they deem as a lack of a “true” partnership mindset. They voiced concerns that CROs mention wanting to be a site partner, but their behaviors may at times suggest otherwise.
- “The best experience is when CRO act as true agents of the sponsor with no hidden agenda.”
- “All we are really asking for is someone committed to listening to us.”
- “When CROs actively keep us at arm’s length from the sponsor, they make us feel like we are just the help. We wish they could be respectful of everyone’s importance in the process. Facilitating direct communication with the sponsor when the need arises enables that.”
Sponsors, too, have work to do to develop a culture of collaboration with their sites, from visiting sites to better understanding their organizational structures and how they work to creating different tiers of site support (e.g., silver, gold, and platinum) based on different site models. But overall, the sentiment of sites regarding sponsors is to simply:
- “Treat us as partners and treat us with respect.”
- “Evaluate what you can do to adopt a ‘true’ site-partner mentality.”
Poor communication flow and lack of responsiveness
A critical component of successful site relationships is communication, which is comprised of people, process, and technology components. On the people side, sites lament that there is no knowledgeable, authoritative, single point of contact on the sponsor/CRO side. Whether it is during the feasibility, budget, and contract negotiation stages, or during study conduct, the ideal site experience includes providing a single point of contact to triage site requests. Sites also long for timely responses to requests and a commitment on the part of the sponsor/CRO to the timelines they impose on sites.
In addition, sites struggle with frequent staff and operating changes for which notifications are delayed or nonexistent. Industry has attempted to combat some of the real-time communication challenges using various portals. However, sites find these impersonal, difficult to navigate, and often static or what they termed “deserted” in terms of meaningful content or their ability to have their questions answered in a timely fashion.
- “Sponsors/CROs push sites to respond in 24 hours but can take 10 days to reply to our requests and this is frustrating.”
- “My best recommendation to improve communication? Just pick up the phone.”
- “Every study will have issues. Managing with integrity, honesty, and transparency with open communication is the only way through vs. finger pointing.”
- “Portals aren’t designed to help sites.”
Nonintegrated technology usage and inadequate support
Continuing the technology theme, sites express frustration with the myriad systems that are imposed upon them. Many sites have their own systems, in which they have made substantial financial investments, to enable them to operate with efficiency and high quality. When sponsors/CROs mandate use of their systems (which often change for each study), all efficiencies are lost, and the site burden is magnified; this increases exponentially as sites work on multiple studies with different sponsors/CROs. Sites bemoan the poor user interfaces with many different technologies and the fact that many vendor systems and devices are not well vetted. Interviewees universally complained about the inadequacy of the help desk services across most vendor systems. This topic garnered more impassioned quotes than any of the other areas discussed in the interview.
- “Who is doing technology well? No one…there is so much tech these days. It takes a long time to figure them out and there are always issues.”
- “Paying sites more to deal with bad technology is sloppy…it just makes us feel less awful about the system but doesn’t fundamentally solve the problem.”
- “Tech support is flat out not provided and working out the bugs of all technology is left to the site to work out. They will tell you there’s a help desk, but they do not know the protocol, they do not work 7×24, they do not support what to do when the data is not captured and are not accountable for its success.”
- “Passwords! Don’t even get me started on this. Changing passwords every 30-45 days across multiple systems is a huge burden.”
- “We’ve never been asked to do UAT of a system before it goes live but that would be awesome.”
Insufficient budgets and repetitive negotiations
Industry has grappled with financial issues for years, so it wasn’t surprising to find this topic appear on the top list of site challenges. Again, some of the primary drivers include people and process issues. On the process side, sites find it difficult to work with generic budget templates that are not tailored to the needs of a specific trial. This is compounded by a larger issue: Budgets fundamentally fail to reflect the “true” site work effort. This leads to endless, often fruitless, rounds of budget justifications which the sites find exasperating and, in the words of some interviewees, insulting. While this disconnect between the sponsor/CRO’s and site’s understanding of what it truly takes to conduct a clinical trial causes inefficiency and waste in the budget negotiation process, at times it is the negotiator’s lack of knowledge and awareness of the site perspective and/or study requirements that may cause even greater pain for the sites.
- “Please just be reasonable with budget negotiations; is saving a few dollars really worth the wasted time and effort when the sponsor ultimately approves our well-justified requests?”
- “Make sure the negotiators are seasoned and understand the delays they are contributing to when they nickel and dime us…remember the goal is about helping patients and accelerating drug development.”
- “Train your budgets and contracts staff on the therapeutic area and indication so they have a clue.”
Inexperienced CRAs and high CRA turnover
Another chronic issue that has plagued industry for years relates to poor quality and high turnover of Clinical Research Associates (CRAs). While there are many highly skilled, high-performing CRAs, this is not the case across the board. Sites understand the challenges that sponsors and CROs face from the shortage of CRA talent and appreciate that this industry-wide issue is somewhat out of their control. Nonetheless, sites believe a much better job can be done to ensure qualifications and address inconsistent monitoring practices across CRAs. Sites feel that they are being tasked with dedicating time to educate and train inexperienced CRAs, which is not accounted for in their budget. Doing the work of the CRAs and redoing work when different CRAs have different interpretations of the protocol and monitoring plans further compounds these frustrations.
- “At the Site Initiation Visit (SIV), we had a full list of questions prepared and the CRA couldn’t answer one. The information wasn’t in the slides, and it took over a month to get answers. When the answers did come, the answers were unfinished or not clear, so we still had a lot of unknowns and no one to get answers from. This is not a one-off experience unfortunately.”
- “What is behind the trend of CRAs not providing their phone numbers? We want to be able to pick up the phone and resolve issues quickly.”
- “I know it is an age-old issue, but a good CRA can make or break the study.”
Cumbersome feasibility and start-up processes
Study start-up is always a complex and fast-paced process. At times it can feel more disorganized than it should, and this exasperates sites. Similar to their communication issues, sites are challenged by unrealistic timelines and turn-around expectations. Sites are expected to respond on demand yet often face a “hurry up and wait” situation for responses from the other parties. In addition, when studies are started before protocol information is finalized, sites are often provided only partial study details that change over time. In addition, many tasks that don’t seem to add value (e.g., pre-study site qualification visits) consume time that could be better spent on other activities from the site’s perspective. Redundant information requests that sites sometimes receive from different members of the same sponsor/CRO study team add another layer of annoyance and inefficiency.
- “What is most helpful during feasibility is having clear details on inclusion/exclusion criteria, clarification of vague points, and clear dates on start-up.”
- “Qualification Visits are ‘a box-checking exercise’ that don’t add value but take time.”
- “When we proactively communicate that our start-up time takes 90 days, don’t hound us every day or every week on what the status is. Check in with us monthly or several weeks before our planned approvals. Hounding us won’t change the facts and just wastes precious time and resources.”
Opportunities for Improvement
These challenges and pain points clearly illustrate numerous opportunities to improve relationships, gain efficiencies, and reduce the investigative site burden. Some opportunities will take time. Others will be more challenging to implement within and across sponsors/CROs and service providers. Each of these topics, however, offers some “low-hanging fruit” or easily implemented actions that can make a substantive impact on site relationships while also enhancing clinical trial productivity. Bolded items in the practical recommendations included for each pain point represent the most often cited solutions offered by interviewees when asked: “If you could recommend just one thing that sponsors and CROs could do today that would really make your life easier, what would that be?”
Overall Sponsor/CRO-Site Relationships
Listening to the sites, being respectful, and facilitating direct site-sponsor communication, when appropriate, are primary strategies for improving site relationships. But these are harder to implement across multiple functional areas with diverse team members who have varying degrees of experience and competencies. Nonetheless, sites appreciate organizations that continue to focus on improvements in these areas. In addition, the same staff at a site are working across numerous studies with different sponsors/CROs, each of which is set up differently, so they need an easy-to-read road map to understand how to work in each setting as they pivot from trial to trial throughout the day. What sites would find immediately helpful would be for study teams to provide an organization chart of study team and vendors with associated contact information so everyone at the site knows who is responsible for what and how to reach them when needed. Back-up contacts are also essential so that sites can always interact with someone with the right knowledge at the right moment.
Communication: Similarly, all parties need to be transparent and responsive in all communications with their investigative sites, specifically, establishing reasonable turnaround time expectations and committing to the same timelines when responding to sites. In essence, be available for sites when they have requests; don’t delay, and don’t pass the buck. Sites may have patients waiting for them, and so responsiveness at all times is crucial. Furthermore, sponsors/CROs should re-evaluate the use of communication portals to make sure they are site-friendly, up-to-date, and provide relevant, easy-to-find information, with proper technical support.
Technology: While technology is a more challenging topic, the more that industry can consider and accommodate the sites’ systems, the less burdensome studies will be to execute. At a minimum, asking sites what systems they already use (e.g., eSource, eReg, eConsent) and allowing as much flexibility as possible are huge opportunities for improvement. Sites are realistic and understand that a fully integrated sponsor and site system and Single Sign On (SSO) for every system may be an unrealistic dream. However, a simple and immediate fix that they would appreciate is extending the timeframe for password expiry to greater than 45 days, such as to 60 or 90 days. Another idea for collaboration as well as improvement is sites being included in User Acceptance Testing (UAT) for various new solutions and study-specific systems. Moreover, providing 24×7 technical support for all systems, along with clear instructions and user manuals, seems a practical and easily implementable solution.
Some of these technology recommendations were further outlined in another recent article published by one of the CRO team members participating in this research.
Budgets/Negotiations: Site respondents interviewed decry the “penny-wise and pound-foolish” approach to budgets often taken by sponsors, as well as by CROs on behalf of sponsors, in which the sites are asked to spend time negotiating and reducing numerous small budget items when larger changes are easily accepted, and (ultimately) it is the total price per patient reimbursement that is the true target. While this may also require a fundamental shift throughout industry, it also offers an opportunity to rethink what is being gained and lost by protracting the negotiation process at a very detailed line-item level. While all parties are aligned with the concept that sites are to be paid fair market value, and transparency of payments is required as part of the US Sunshine Act reporting, there is a degree of minutiae in the negotiation process that sites feel is taken too far and requires additional time that adds little to no value. Furthermore, sites feel that they are not being fairly compensated for all the activities they are asked to perform. Ensuring that budget negotiators are educated about the therapeutic area and study indication can also go a long way to avoid misunderstandings about the true work effort when conducting increasingly complex trials. But implementing telephone versus email negotiations is at the top of sites’ desires. Eliminating time-wasting eMail exchanges and escalations which must ultimately be resolved through telephone discussion would go a long way to ease the burden of this process, minimize frustrations, and accelerate study start-up times.
CRA Quality/Turnover: As noted above, sites recognize the importance of interacting with the same well-trained CRA who follows a consistent approach. They also recognize that this is not always practical or feasible given the industry-wide CRA shortage. However, at a minimum, sponsors/CROs should strive to develop and retain CRA talent, to minimize turnover as much as possible, and to ensure that the CRAs who conduct Site Initiation Visits (SIVs) are well-versed in the protocol and prepared to answer questions about the trial at the SIV and throughout the study. If the CRA cannot answer these questions immediately, then sponsors and CROs should insist on timely responses to site questions or face the consequences of disengaged and frustrated sites. At a more practical level, site staff advocate that all CRAs provide their phone number on eMail signature lines so they can quickly contact them. This appears to be a seemingly intuitive recommendation: If the CRA is to be the single point of contact for the site, shouldn’t the site be able to reach them at all times?
If not, then perhaps it is time to change the long-established model that the CRA is designated as the site’s single point of contact. Sites applaud the sponsors and CROs who have implemented new roles such as site relationship managers (or equivalent titles). Regardless of the roles, sites encourage all sponsor/CRO staff to ensure their contact information is easy to find whether it is on an organizational chart or eMail signature.
Feasibility/Start-Up Process: Last but not least, site staff offered many suggestions to accelerate site activation activities. Sites hunger for more information earlier in the trial feasibility assessment process. While a robust protocol synopsis and draft budget are ideal at a minimum, sites require clarity around the patient population (i.e., clear details on inclusion/exclusion criteria) and clear dates around planned start-up times. They also support holding feasibility webinars, instead of sending redundant questionnaires, to foster good information exchange about the study design and clarify operational aspects that are often not well outlined in the synopsis, but which greatly impact study execution success and resource planning. However, the biggest opportunity is to eliminate the need to complete redundant information asked on feasibility questionnaires (e.g., pre-populate the questionnaire with already-known site information).
On the start-up front, site staff challenge the industry to rethink the necessity of pre-study Site Qualification Visits (SQVs, or an equivalent term defined by the sponsor/CRO). Sites recognize that regulations and ICH GCP guidance require sponsors to select investigators qualified by education, training, and experience. With more site performance databases that compile and showcase site experience available to industry (for example, sponsor/CRO-specific and vendor-supported systems such as the Shared Investigator Platform [SIP], Investigator Databank, Phesi, and Citeline), sites question whether sending CRAs out to confirm already known site qualifications is a value-added step in the process or whether it is more of a “box-ticking exercise,” and if the time has come to transform these practices.
Having well-documented site start-up processes and timelines for each site will also go a long way toward eliminating the burdensome check-ins on status updates and the need to request information that is already clearly captured elsewhere.
Summary and Conclusions
Achieving the illustrious “sponsor/CRO of Choice” site designation remains elusive for many organizations. Technology investments appear to have compounded chronic site frustrations, and well-intentioned efforts don’t appear to be moving the needle in terms of effective site relationships. However, the results of this research effort uncover some surprisingly practical and actionable solutions.
Contained within many of these ideas is the old-fashioned, nearly effortless activity of good communication practices and picking up the phone. Simply speaking with sites and asking them about their needs and recommendations can be a highly effective means of uncovering relatively easy solutions and becoming a sponsor/CRO of choice. Furthermore, phone (or web meeting) discussions allow issues to be addressed promptly and eliminate misunderstandings that occur through endless eMail exchanges. While these interviews were conducted in the context of a specific research project, it is not hard to see where these solutions can address many challenges throughout the clinical trial lifecycle, from the feasibility assessment process through budget and contract negotiations, SIVs, and study conduct. Going “back to basics” takes a little time, effort, and technology investment, and yet can have a far-reaching impact on the sites’ experience and productivity, and ultimately gives sites additional time for their most critical activity: caring for patients.

