University of Pittsburgh School of Medicine
obel Prizes in Medicine and Physiology were awarded in 1966, 2005, 2008, and 2020 to scientists who discovered that cancers can be caused by infectious agents, which opened opportunities to prevent those cancers with vaccines. Vaccines against hepatitis B virus (HBV), which causes liver cancer, and human papilloma virus (HPV), which causes cervical cancer, have already saved millions of lives by preventing these lethal cancers. Vaccines against other known cancer-causing viruses are under development. Only one in 10 human cancers is known to have infectious etiology, leaving the most common cancers, such as melanoma, breast, prostate, pancreatic, colon, lung, head and neck and brain cancer, T and B cell lymphomas, and multiple myeloma seemingly without a possibility for prevention by vaccination. It is now clear, however, that the immune system can recognize nonviral cancers by their expression of molecules that, while not viral or bacterial in origin, are nevertheless foreign to the immune system due to differences in expression on cancers compared to normal cells. These molecules, known as tumor antigens, are candidates for preventative vaccines for nonviral cancers.
Cancer-Causing Viruses and Conventional Vaccines for Cancer Prevention
Seven viruses and one bacterium have been identified so far as causes of multiple human cancers (Table 1). These viruses infect a large percentage of the population globally, and while not every infection results in cancer, enough do to account for hundreds of thousands of cancers annually. Because who will clear the infection and who will develop cancer cannot be predicted, general vaccination early in life, before exposure, has been shown to reduce the cancer risk in the population greatly. Around 600,000 cases of cervical cancer are diagnosed each year, 98% of those caused by HPV. The data obtained on 60 million vaccinated individuals 8 years post-HPV vaccine approval and implementation showed that infections decreased by 83% in girls vaccinated between 13 and 19 years of age. The most recent follow-up study on 1.7 million women worldwide showed a 90% reduction in cervical cancer if vaccinated. Another significant benefit of the vaccine is the potential to prevent HPV-positive head and neck cancers, which are more prevalent in men, warranting vaccination of both sexes. A similar story had already unfolded with HBV vaccines, where neonatal vaccination greatly lowered the risk of developing liver cancer.
These cancer-prevention vaccines are the conventional type, based on viral antigens and inducing responses to viruses and virus-infected cells. They prepare the immune system to counter the virus, like vaccines against polio, chicken pox, or mumps. Given the successes of HBV and HPV vaccines, it is disheartening that more such vaccines have not yet been developed. While research and development of vaccines requires time, the major reason that more vaccines have not been developed is lack of investment of sufficient resources to speed up this process. This was amply illustrated by how fast COVID-19 vaccines were developed when scientists, governments, and pharma decided to pull their unique resources together. The COVID pandemic, which is subsiding thanks to vaccination, has claimed 6,200,000 lives so far. Cancer has been an ongoing pandemic claiming 10,000,000 lives each year. The time is right for a similar all-out effort and investment to develop preventative vaccines for both viral and nonviral cancers.
The Long Road to Preventative Vaccines for Nonviral Cancers
Development of preventative vaccines for nonviral cancers has been hampered by incorrect assumptions.
One was that there were no antigens against which a vaccine could elicit a response against cancer that would not react against normal cells. This assumption persisted long after numerous tumor antigens were discovered on multiple human tumors and shown to be effective at eliciting immunity against tumors and not normal cells.
The other assumption was that tumor antigens were self-antigens that would either induce immunological tolerance (no reaction against self) or elicit only weak immunity that could not stand up to cancer challenge. Again, many self-derived human tumor antigens were characterized as being capable of eliciting tumor-specific immunity without any autoimmunity.
Another assumption was that trials of preventative vaccines would require thousands of participants and observation periods of 20-30 years to evaluate their preventative potential. A lot has been learned about cancer risks in specific populations due to family history, specific genetic mutations (e.g., BRCA1 and BRCA2), or specific lifestyles, such as heavy smoking. Sophisticated algorithms for prediction of risk have been developed. They can greatly facilitate the design of preventative cancer vaccine trials that can be conducted in a relatively small number of individuals with a well-defined risk of developing cancer, and within a relatively short period of time.
Failed Attempts to Make Cancer Vaccines Effective in Cancer Therapy
Expecting cancer vaccines to be effective as therapy was without precedent. Except for the rabies vaccine, which is administered post-infection and immediately post-exposure, all other vaccines prevent rather than cure disease. Cancer vaccines based on some of the most promising tumor antigens, including viral antigens on virus-induced cancers, were required initially to be tested in the setting of metastatic disease and later in the setting of minimal residual disease. This was based on another wrong assumption: that in cancer, unlike in infectious disease, there may be a longer window of opportunity to intervene—the period between the removal of the primary tumor and disease recurrence, which can take from months to years. It took many years of vaccine development, optimization with new antigens, new adjuvants, and new delivery systems to realize that the failure to change the clinical course of disease with vaccines may not be a matter of bad vaccines. Even HPV vaccines, which work so well in preventing HPV infection and cervical cancer, were ineffective once cancer had become established. Animal models taught the same lesson: vaccines were highly immunogenic in healthy mice and could protect from tumor challenge but had very little or no activity against established tumors. Therefore, applying vaccines early in tumor development is preferable to waiting.
Over 30 years of outstanding science, the best immunology, and the newest technologies failed to produce effective therapeutic vaccines. However, this work yielded major discoveries about cancer and the immune system. It is now clear that a tumor diagnosis is an indication of tumor escape from immune control and that the immune system at that point is profoundly modified and suppressed by the tumor and other suppressive cells and factors in the tumor microenvironment. The many mechanisms of immune suppression, so-called checkpoints, provided new targets for therapy. Blocking CTLA4, one of the first checkpoints on T cells that tumors use to suppress their activity, led to reactivation of T cells in melanoma patients and in some to a complete elimination of all tumors. This provided the first example that the immune system in some instances can be reengaged in the fight against cancer, and the first example of curative immunotherapy, which in 2018 earned one more Nobel Prize in Immunology.
Preventative Vaccines May Finally Be Here
One of the major roles of the immune system is to survey for the danger that tumors present and to try to prevent it. Immune surveillance of cancer is most effective in eliminating early lesions or keeping them from progressing to cancer. Many human tumors have well-defined premalignant stages and advanced technology has been developed, such as mammograms, endoscopic cameras, CT and MRI scanners, and specific physical exams and blood tests (e.g., Pap smear for cervical cancer, colonoscopy for colon cancer, PSA for prostate cancer) for their detection. Some are surgically removed, while others continue to be monitored.
A diagnosis of premalignant lesions is an indication of immune escape, and it carries an increased risk of cancer. Most individuals with premalignant lesions are immunocompetent and would be expected to respond to vaccines better than cancer patients. Boosting immunosurveillance at this stage with vaccines against antigens expressed on premalignant lesions would be expected to result in either their complete clearance or interception of their progression to invasive cancer (Figure 1). Fortunately, many of the shared tumor antigens in vaccines that failed as therapy are expressed on premalignant lesions, and many vaccines are ready to be tested in that setting.
The most important test of preventative vaccines is the safety of the immune response they elicit. In several preclinical and clinical studies, these vaccines have been shown to be more immunogenic in premalignancy than they had been in cancer. The first of these trials used an HPV vaccine in women with cervical and vulvar intraepithelial neoplasms, CIN and VIN, to prevent progression to cancer. The vaccine was immunogenic, safe, and effective at eliminating CIN and VIN lesions.
The first trial of a vaccine for nonviral cancers, run between 2008 and 2012, was of a MUC1 vaccine given to individuals with a history of advanced colonic adenomas, precursors to colon cancer. The vaccine was highly immunogenic, generating immunity without any autoimmune or other side effects. A follow-up randomized placebo-controlled trial run between 2014 and 2020 also showed indications that the vaccine could be effective. Vaccine responders had a 38% reduction in adenoma recurrence. This trial enrolled 110 individuals and all the results were obtained in 5 years, showing the potential of testing preventative vaccines in a smaller number of trial participants, and in a reasonably short time.
Another clinical trial is ongoing and is testing an hTERT vaccine in women diagnosed with ductal carcinoma in situ (DCIS), a precursor to invasive breast cancer. A preventative vaccine will soon be tested in Lynch syndrome, which causes large polyp burdens and has a high risk of progression to colon cancer. Several antigens with frame-shift mutations were identified in Lynch colonic polyps and are being tested currently in preclinical mouse models to choose the best candidates for vaccines.
Other planned trials are with mutated KRAS vaccines for pancreatic cancer prevention in individuals with preneoplastic pancreatic intraepithelial neoplasms (PanIN), multiantigen vaccines for colon cancer prevention, and anti-Mullerian hormone receptor II vaccines for ovarian cancer prevention.
These completed, ongoing, or planned trials are showing the path for testing and implementation of preventative cancer vaccines, first in individuals with premalignant lesions, where some immune responses are already compromised but where safety and efficacy can still be properly tested, and then in individuals without any lesions but at an increased risk for cancer.
Preventative Cancer Vaccines: Risk-Benefit Versus Risk-Risk
In 2004, scientists, cancer advocates, and regulatory and government agencies convened in Washington DC to discuss cancer prevention. Attending the meeting was journalist Clifton Leaf, who had just published a widely read article in Fortune magazine, “Why We’re Losing the War on Cancer and How to Win It.” As comments were requested around the conference table, almost everyone addressed the “risk-benefit ratio” of various preventative approaches. Since benefits were still to be shown, the risk concerns prevailed. When his turn came, Leaf made the brilliant suggestion that we change the “risk-benefit” discussion to a “risk-risk” discussion, the risk of doing something versus the risk of doing nothing. Globally, each year there are over 19 million cases of cancer and 10 million deaths.
In addition to the agony of cancer diagnosis and the morbidity of most cancer treatments, patients and their families suffer financial hardships. Healthcare budgets of nations are already straining under the costs of cancer care, and those are likely to continue to increase as the incidence of cancer increases. It is thus a smart humanitarian and economic policy to invest in cancer prevention to a much higher degree and particularly in vaccines that are safe, practical, effective, and inexpensive and can be implemented around the world, including in many low-income countries. The risk of investing in prevention and vaccines would be that some approaches might fail or some vaccines may be ineffective or toxic; in addition, this would require large financial investment, primarily from wealthier countries. The risk of not investing in prevention and vaccines will be the continued increase in cancer burden and human suffering and increased financial strain on world economies, which will eventually become unsustainable. Clearly the latter risk is the one we should not take.
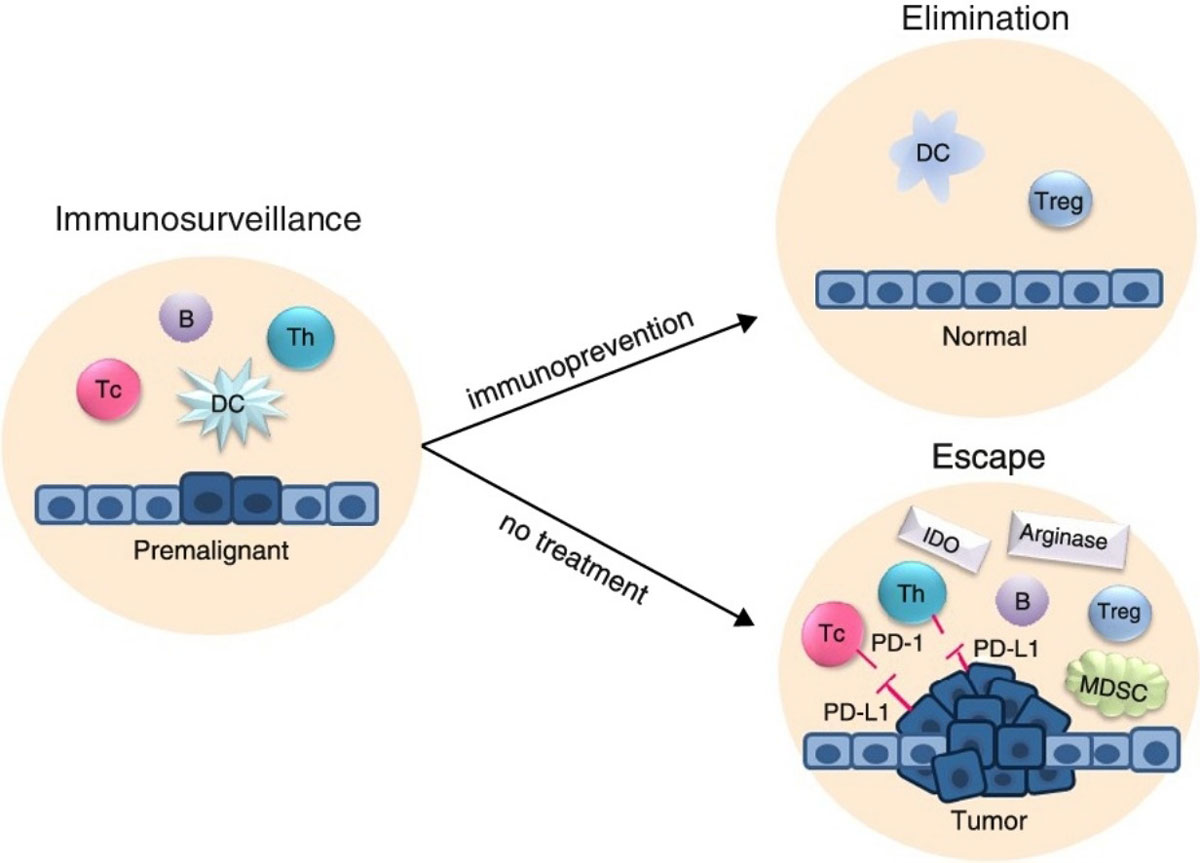
Figure 1. Importance of developing preventative cancer vaccines for strengthening cancer immunosurveillance. As healthy tissue under various carcinogenic influences starts to undergo premalignant changes, immune cells are alerted to come to the site, generate large numbers of antigen-specific cells and antibodies, and eliminate abnormal cells. Left untreated, these lesions give rise to invasive cancer and an establishment of an immunosuppressive tumor microenvironment. If the immune response is strengthened with a preventative vaccine, premalignant cells are eliminated, and healthy tissue restored.
Tc, cytotoxic T cell; Th, helper T cell; B, B cell; DC, dendritic cell; Treg, regulatory T cell; MDSC, myeloid-derived suppressor cell; IDO and Arginase, immunosuppressive soluble factors; PD-1 and PD-L1, immunosuppressive cell surface molecules.

