Meeting Highlights: DIA Europe 2021
General Principles on Planning and Designing Multi-Regional Clinical Trials
AstraZeneca
Merck
Novo Nordisk
GSK
Novo Nordisk
AbbVie
Pfizer
@EFPIA
ow does industry perceive the impact of ICH E17 Multi-Regional Clinical Trial (MRCT) guideline in some key international countries? Are the guideline recommendations fully utilized, and how are regulatory authorities being perceived in implementing them? This article shares the results from a recent industry survey about how implementation of the E17 guideline is progressing in seven countries.
Background: Why Did the E17 Guideline Come About?
Industry Survey
The survey comprised three sections:
- Section A: to gauge the activity level and awareness or understanding in industry,
- Section B: to gather data on implementation of different aspects of the guideline to guide training and advocacy, and
- Section C: to understand industry views on the benefits of and hurdles to implementation.
The survey readout helped identify key challenges and guide training and advocacy activities to further ICH E17 guideline implementation.
Results
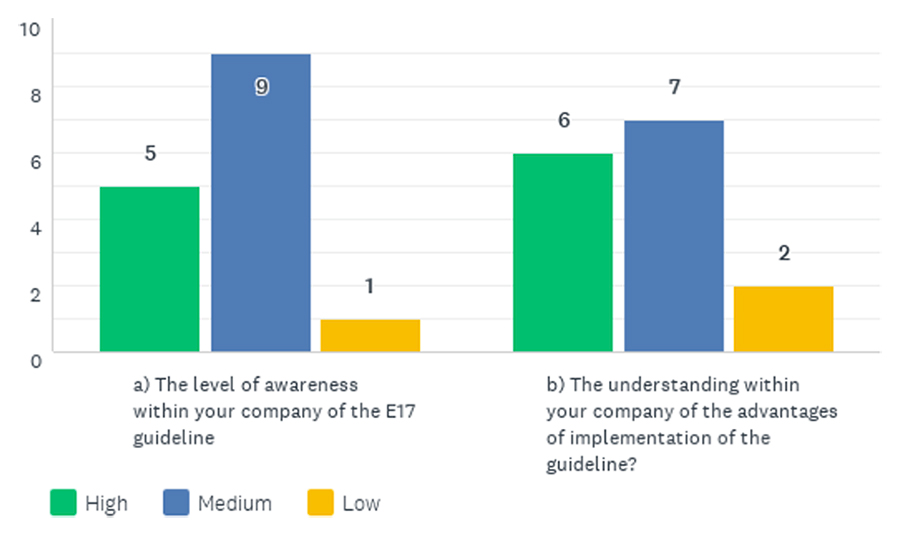
Fifteen member companies completed the survey. Most respondents (14 of 15) reported that awareness of the ICH E17 guideline was medium or high within their company. Similarly, 13 companies rated their understanding of the benefits of implementing the guideline as medium or high (Figure 1).
It was encouraging to see that most companies (10 of 15) were willing to modify their processes to adapt to the ICH E17 guideline, and an additional four companies said they are somewhat willing to. This shows a willingness to engage more under the right circumstances.
Country Focus: Discussions with Regulators
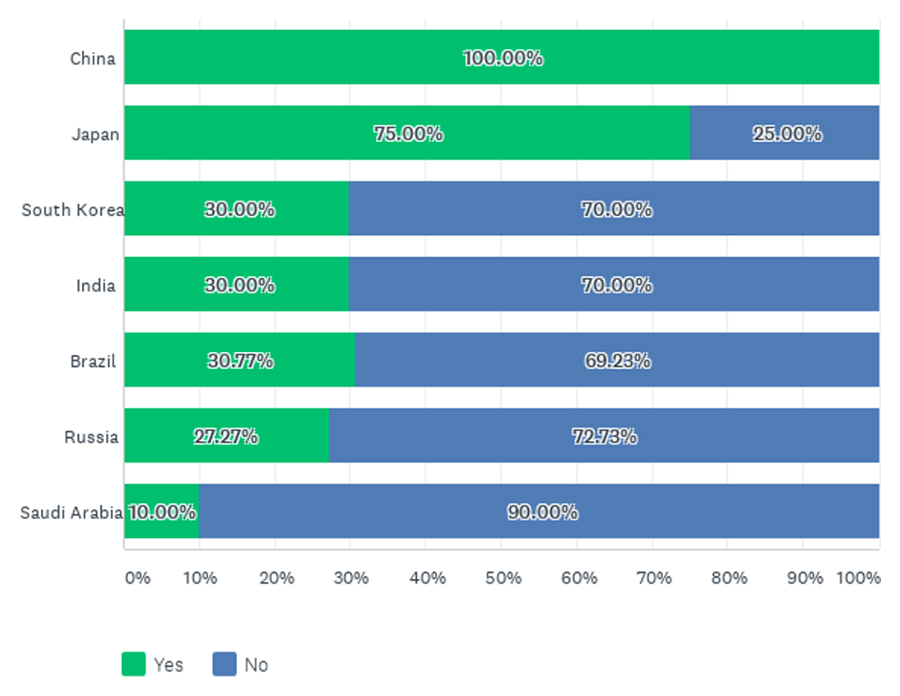
When asked about interactions with regulatory authorities regarding their development programs since finalization of ICH E17, all respondents confirmed they had interacted with China’s National Medicinal Products Administration (NMPA), and most companies had interacted with Japan’s Pharmaceuticals and Medical Devices Agency (PMDA). For the other countries of interest, such interactions were only reported by around one-third of these companies, except for Saudi Arabia where only one company reported interactions (Figure 2).
Requests for Local Clinical Data and Different Therapeutic Areas
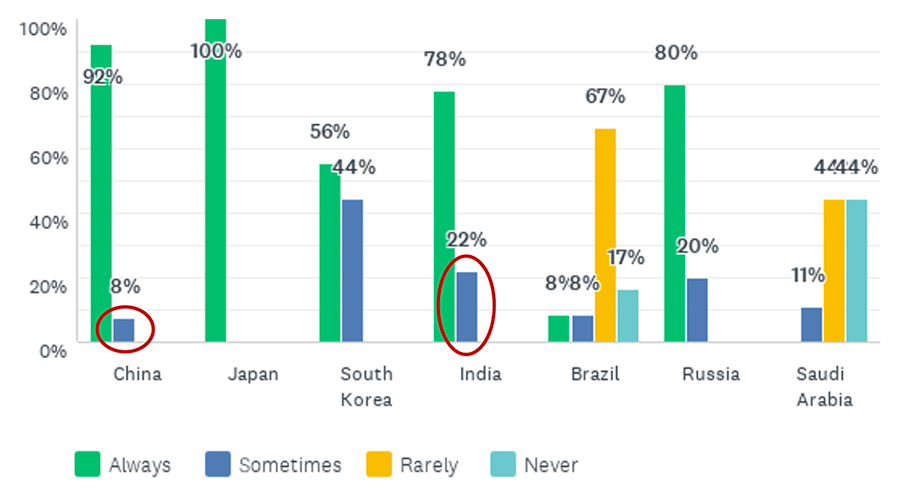
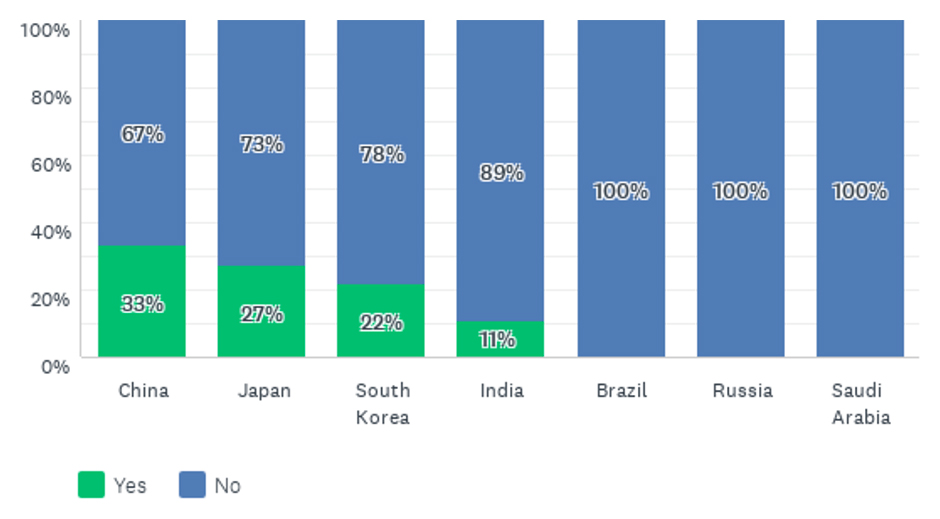
These findings are linked to the answers to the question about whether a country requests local clinical data. Japan’s PMDA and China’s NMPA were often said to always request local trials (Figure 3).
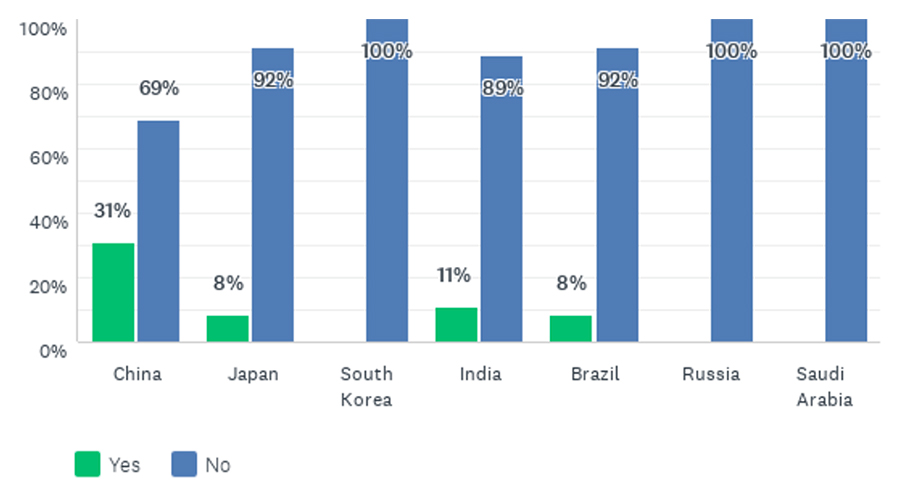
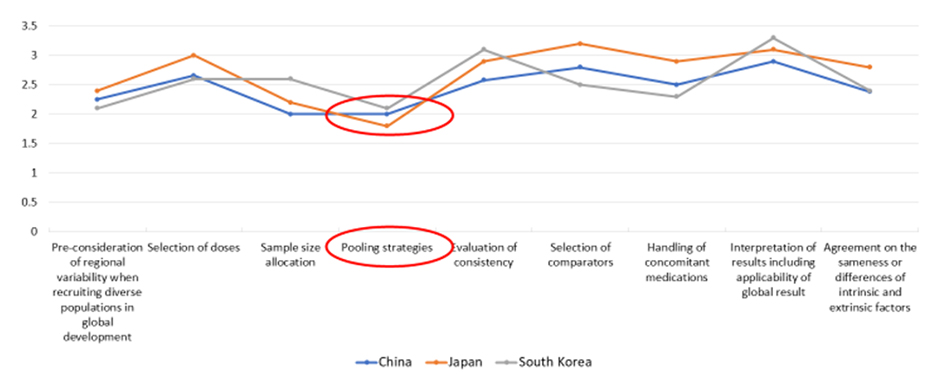
The survey also explored greater insight into a range of different aspects covered by the guideline. Respondents were asked to rate how well various aspects had been applied in practice on a scale of one to four (four being best) in the different countries. Pooling strategies—the prespecified pooling of regions or subpopulations that may help provide flexibility in sample size allocation to regions, facilitate the assessment of consistency in treatment effects across regions, and support regulatory decision-making—emerged as an aspect where implementation could be improved in China, Japan, and South Korea (Figure 6). Can pooling of patients from Japan, South Korea, and China into an East Asian region be considered, provided that the ethnic factors are adequately understood and comparable?
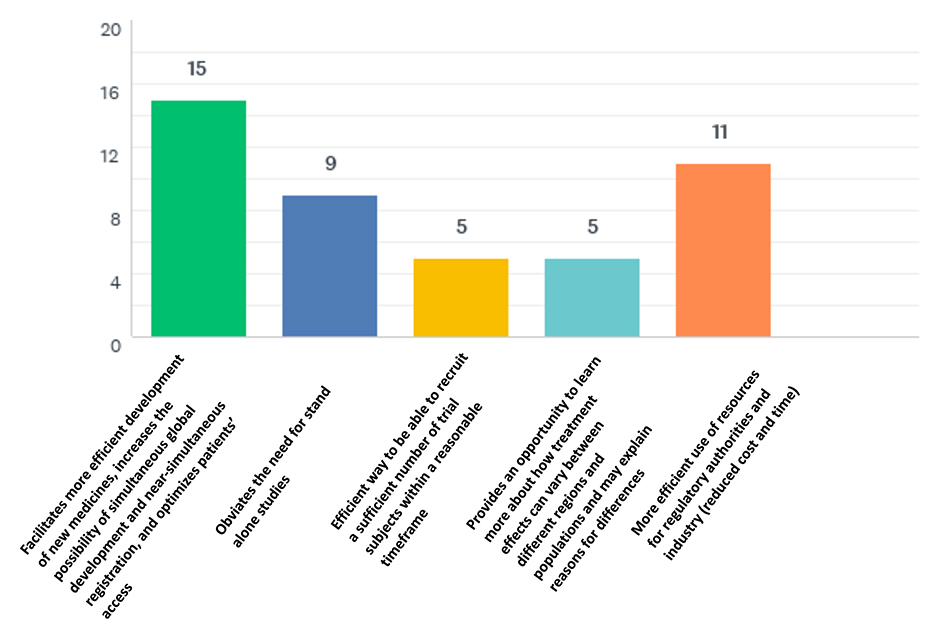
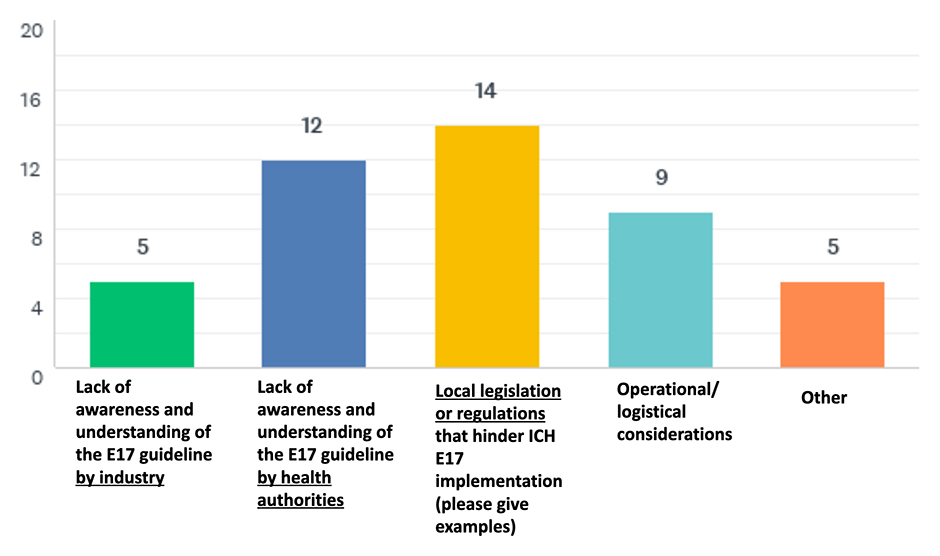
Discussion and Conclusions
- There is an opportunity to promote awareness of ICH E17 training materials, which are not widely used by industry respondents to this survey. This would also widen company understanding of the guideline beyond those working directly with clinical trials.
- However, training materials are considered very general by those respondents who use them; these should be revised in the future, including adding more case examples.
- It is probably too early to see an impact on requests for local clinical trials since implementation is still ongoing in some countries.
- The need for a better understanding of the ICH E17 guideline by regulatory authorities and industry remains.
- Challenges (such as pooling strategies) remain.
- Finally, local legislation and/or regulation are perceived as a barrier to full implementation of the ICH E17 guideline.
The ICH E17 guideline is undoubtedly an important tool in global drug development. Despite sub-optimal use of pooling strategies, new medicines are getting approved in Japan and South Korea at about the same time as in other major markets, and China is taking a leap toward this ambition.
We encourage all stakeholders to strive for full implementation of the ICH E17 guideline recommendations, including the ability to pool subregions. This will ultimately support further development of MRCTs and their acceptance by regulatory authorities globally.

