Around the Globe
Fresenius Medical Care Asia Pacific
n January 23, 2020, Wuhan and other cities in Hubei Province, China, began more than 70 days of mandated lockdown due to the outbreak of Coronavirus Disease 2019 (COVID-19). During this period, most other cities in China were also impacted, especially hospitals and clinics mainly focused on fighting COVID-19. Most ongoing clinical trials were suspended.
Organizations surveyed included sponsors, research centers, system providers, regulators, and other types, and encompassed both drug and device clinical trials. Respondent clinical trial team members included investigators, research center staff, patient recruitment staff, clinical research coordinators, CRAs, project managers, data managers, programmers, and statisticians.
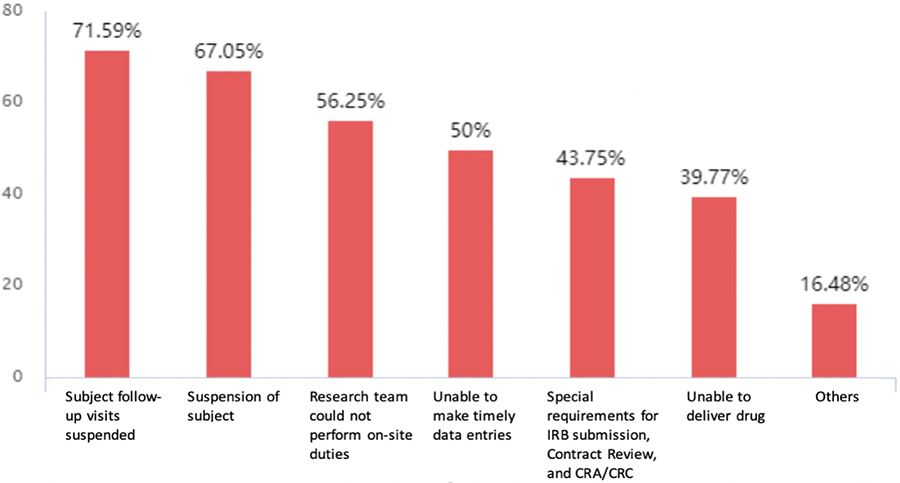
Among the 176 responders, 131 (74.4 percent) stated that their projects had been suspended primarily due to failure to conduct patient follow-up visits (71.6 percent), suspension of patient recruitment (67.1 percent), inability to make timely data entries (50 percent), and inability to make timely delivery of the study drugs (40 percent).
Remote Data Monitoring
As of February 27, users from 63 sponsors were granted access to this system. Clinical data from a total of 2,885 patients in 154 clinical projects across 16 hospital departments have been verified. Issues found across 52 remote monitoring projects included failure to conduct follow-up visits on the protocol schedule (117 times), failure to take the drug on the protocol schedule (105), missing electronic Case Report Form (eCRF) data (79), failure to deliver the drug on the protocol schedule (74), and inconsistency between the CRF and source data (69 times).
Outcomes and Assessments
An ePRO is not only easier for a patient to complete remotely compared to a paper PRO, but it may also improve data quality. A case study comparing the compliance rates for 80 patients keeping either paper or electronic diaries showed that the mean compliance rate was 94 percent (95% CI: 92%-96%) in the electronic diary group but only 11 percent (95% CI: 8%-14%) in the paper diary group.
The most popular ePRO platforms are currently mobile application (APP) and the WeChat Mini-Program. Dong Ma (Taimei Medical Technology) presented an ePRO APP application case. This ePRO APP comprises the eConsent, eVisit, eSource, and EDC functions. Patients can download the APP and register through an invitation code sent by the investigator. The patient must watch an introductory video and pass a test on the informed consent process before signing the electronic Informed Consent Form. After that, the patient enters the ePRO system and completes reports according to protocol requirements. The system sends out reminders for data submission and queries raised by the investigator, and intelligently detects and masks patient privacy information.
Intelligent Systems and Platforms
Such intelligent platforms can also acquire patient data from different scenarios. For instance, remote data monitoring systems including devices for file scanning and the Patient Management APP allows patients to self-report data, and the WeChat Mini-Program platform features ePRO, visit reminder, and DTP functions. Furthermore, systems are connected with analytic tools that can perform screening, variant selection, variant calculation, and data preview (statistic description). Some full-process intelligent clinical platforms also provide safety, pharmacovigilance, regulatory submission, and AI image evaluation features.
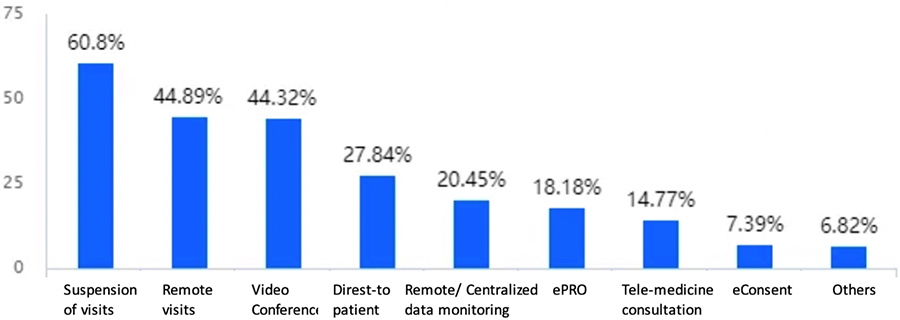
By employing remote clinical trials to address the challenges of conducting traditional clinical trials posed by the COVID-19 pandemic (impacting on-site subject visit, data monitoring, and drug delivery), sponsors and CROs have been able to maintain their business continuity in China. However, because such trials are just getting started in China, it is important to optimize the impact of these technologies and services and pertinent regulations should evolve to appropriately support these developments.

