From Market Authorization to Patient Access: How Long is Too Long?
EFPIA Patients W.A.I.T. Indicator
EFPIA Patients W.A.I.T. Indicator
Edith Frenoy
Director, Market Access
European Federation of Pharmaceutical Industries Associations (EFPIA)
@EFPIA
Background and Scope
R
egulatory approval (marketing authorization) is necessary for drug launch but is not sufficient to guarantee patient access to a licensed medicine. There is a time lag between a marketing authorization and patient access. The question of how long that lag is matters a great deal to patients. EFPIA sought to answer this question with empirical data.
The EFPIA “Patients W.A.I.T.” (Patients Waiting to Access Innovative Therapies) indicator provides a benchmark of the rate of availability and waiting times for new medicines in European countries. For new medicines (defined as medicines that include a substance which has not been previously available in Europe) up to a specific cut-off date, the report shows the following within a (rolling) three-year or four-year cohort:
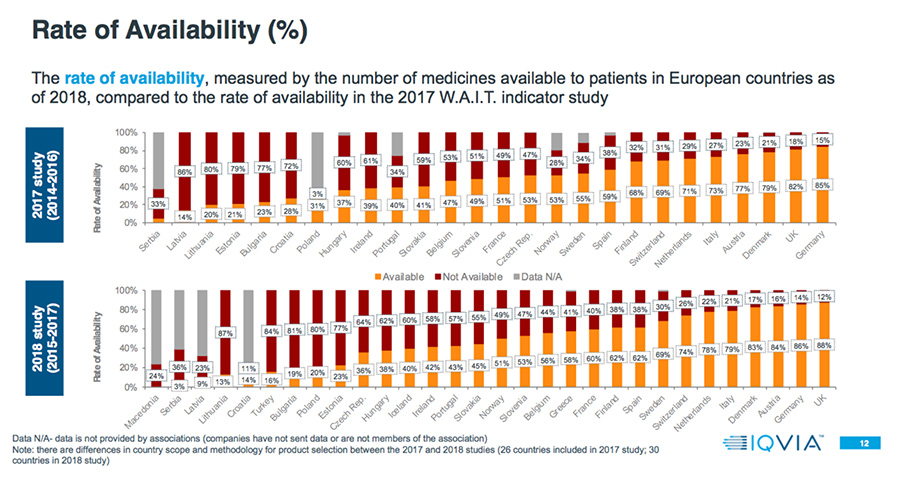
- The rate of availability, measured by the number of medicines available to patients in European countries.
- For most countries, this is the point at which the product gains access to the reimbursement list (see exceptions/explanations in callout box below).
- In some countries, products are available with special reimbursement conditions (see exceptions/explanations in callout box below).
- The rate of availability in a country does not necessarily indicate medicine uptake, as some medicines may be available in a market with no uptake (no sales or no volume).
- The average time between marketing authorization and patient access, measured by the number of days elapsed from the date of EU marketing authorization (or effective marketing authorization in non-EEA (European Economic Area countries) to the day of completion of post-marketing authorization administrative processes. Waiting times reflected in the Patients W.A.I.T. Indicator include any delay, whether attributable to companies or to competent authorities.
The analysis is based on information gathered from EFPIA member associations, who either refer to information available from official sources or gather this information directly from member companies.
Data Sources and Development Methodology
IQVIA supported EFPIA for development of the 2018 W.A.I.T. indicator. This report is based on 121 products approved by EMA between 1 January 2015 and 31 December 2017 (excluding four products withdrawn from 2015-2017), and examines 30 EU and non-EU countries. The WAIT indicator also provides an analysis for a subset of products such orphan products, oncology products, and combination products. The full report is available on the EFPIA website and features detailed explanations on methodology and definitions.
Results: Rate of Availability
The 2018 report shows that:
- Patient access to new medicines is highly varied across Europe, with the greatest rate of availability in Northern and Western European countries and lowest in Southern and Eastern European countries.
- In some countries, up to 30 percent of products are available and reimbursed but with specific conditions.
- In 78 percent of the countries, the rate of availability is lower for Orphan drugs compared to all products approved in 2015-2017.
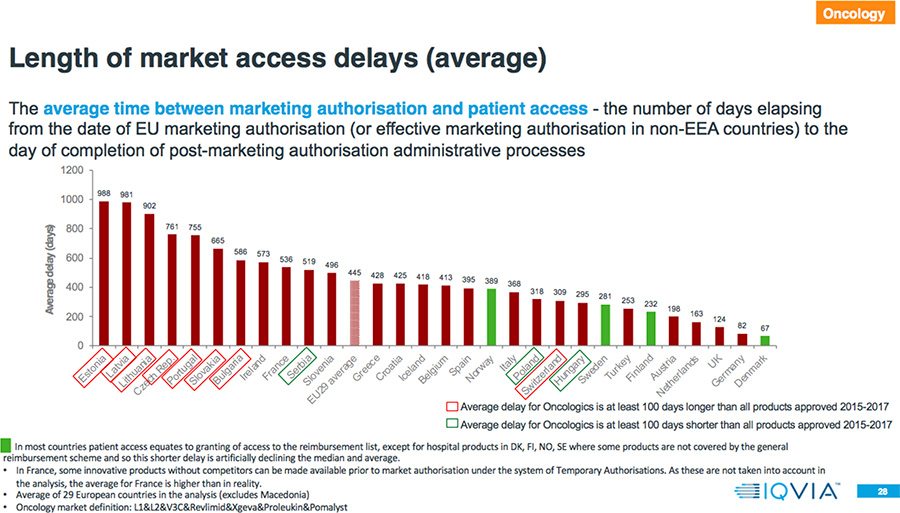
- In 78 percent of the countries, the rate of availability is higher for Oncology drugs compared to all products approved in 2015-2017.
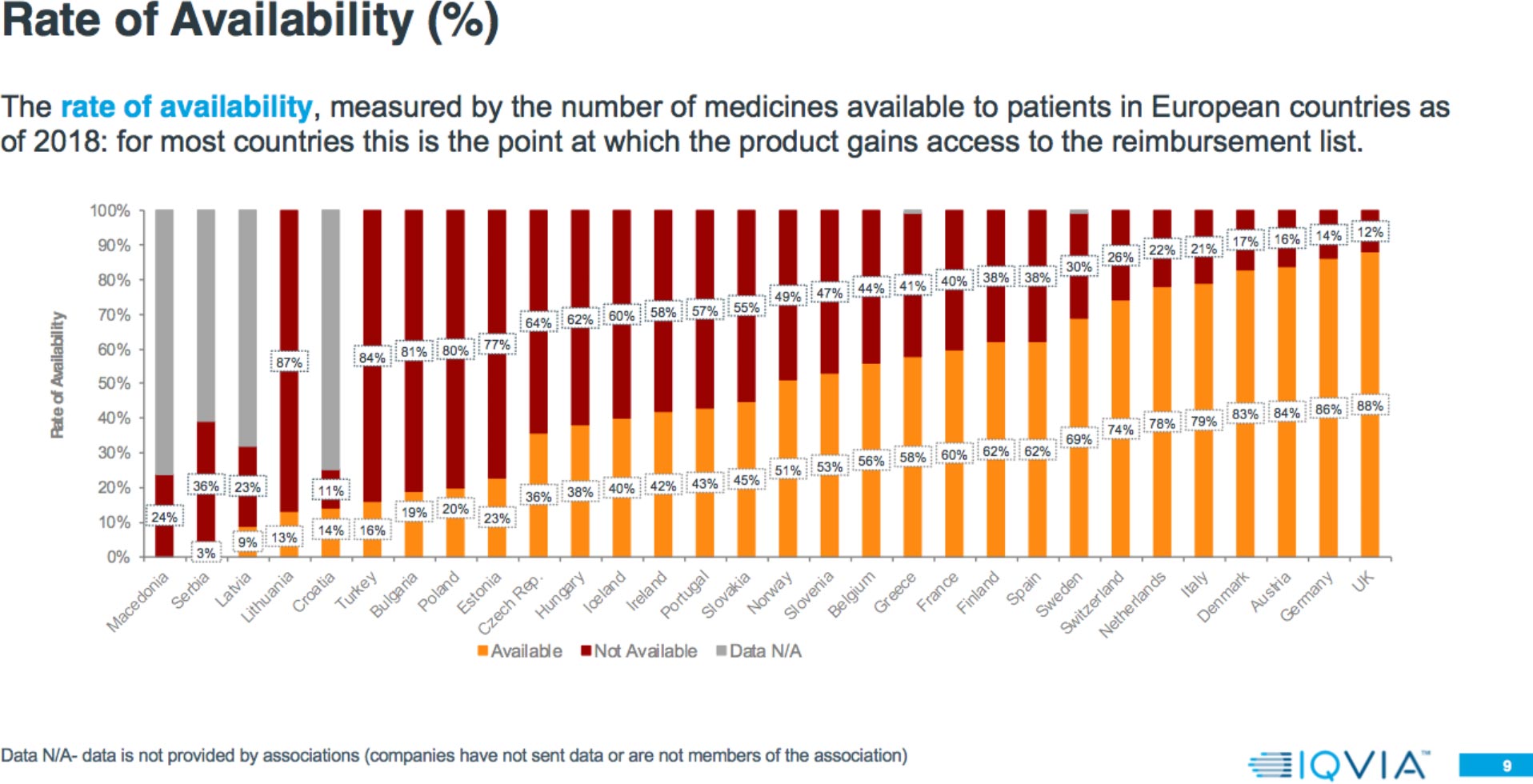
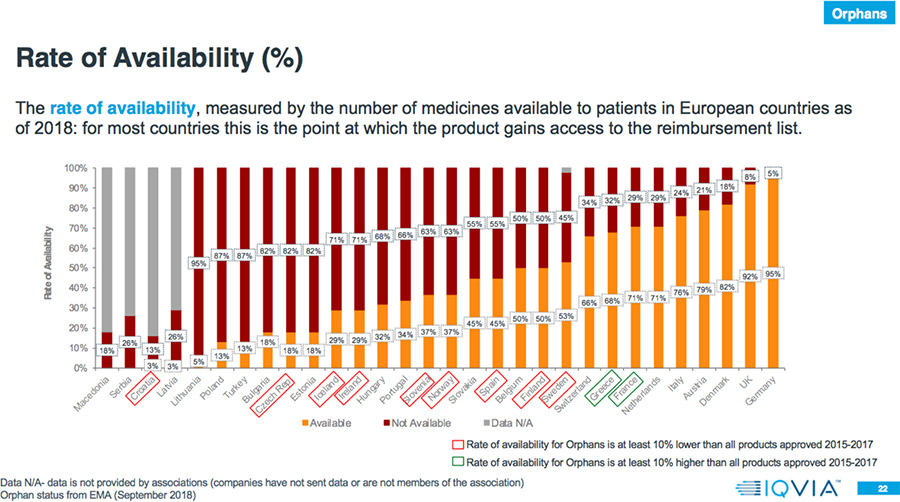
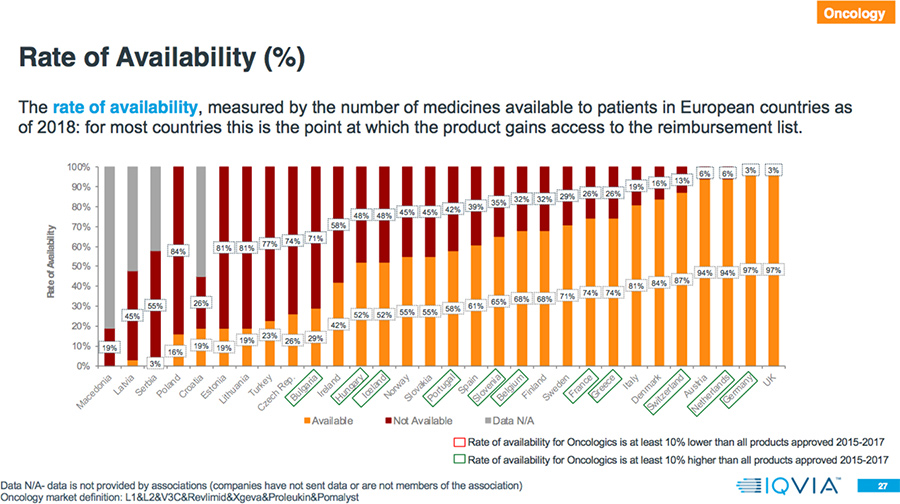
Results: Market Access Delays
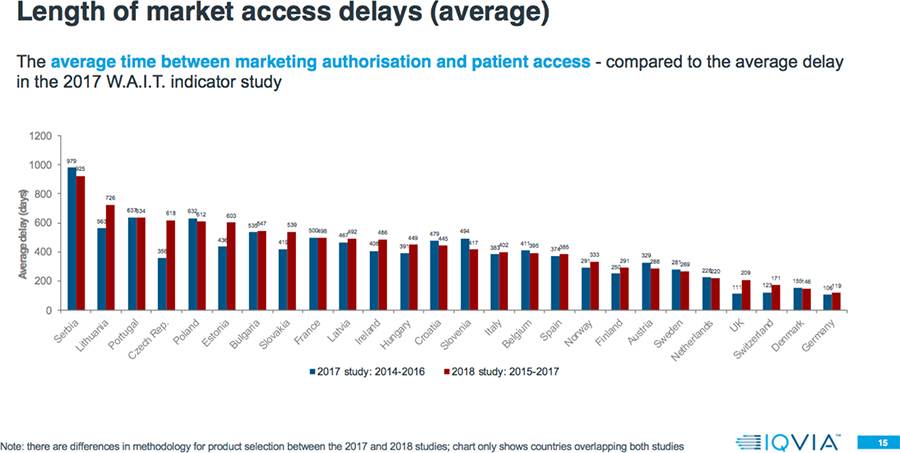
- The average delay between market authorization and patient access can vary by a factor greater than seven-fold across Europe, with patients in Northern/Western Europe accessing new products from 100-200 days after market authorization, but 600-1000 days from market authorization for patients mainly in Southern/Eastern Europe.
- Even within a country, there is a large variation in the speed of patient access to different products. Often the level of variation within a country is greater than the variation between countries; e.g., shortest versus longest delays in Estonia is 21 days versus 1443 days; zero (0) to 1321 days in Ireland; and 33 versus 1383 days in Austria.
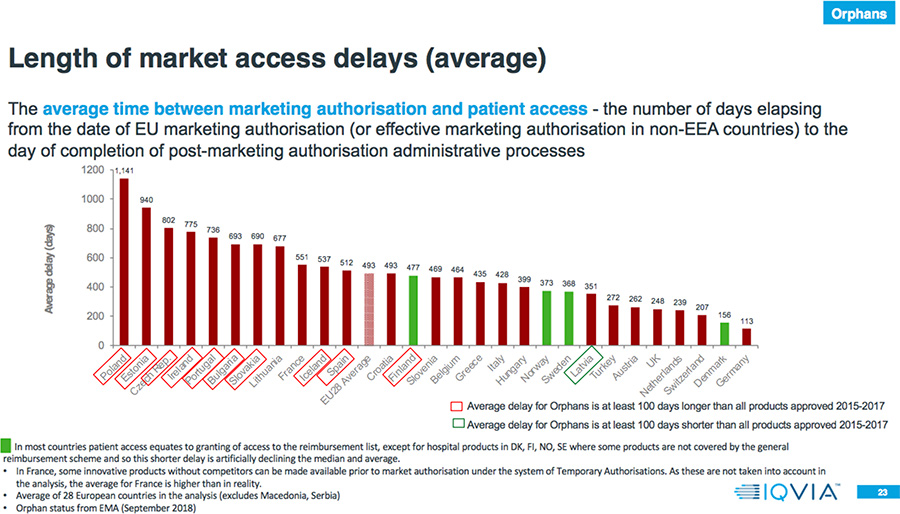
- 80 percent of the countries have a longer average delay to EMA authorization for Orphan drugs compared to all products approved 2015-2017.
- The average delay between market authorization and patient access for Orphan drugs is between four months and three years.
- The average delay between market authorization and patient access for Oncology drugs is between two months and more than two and one-half years.
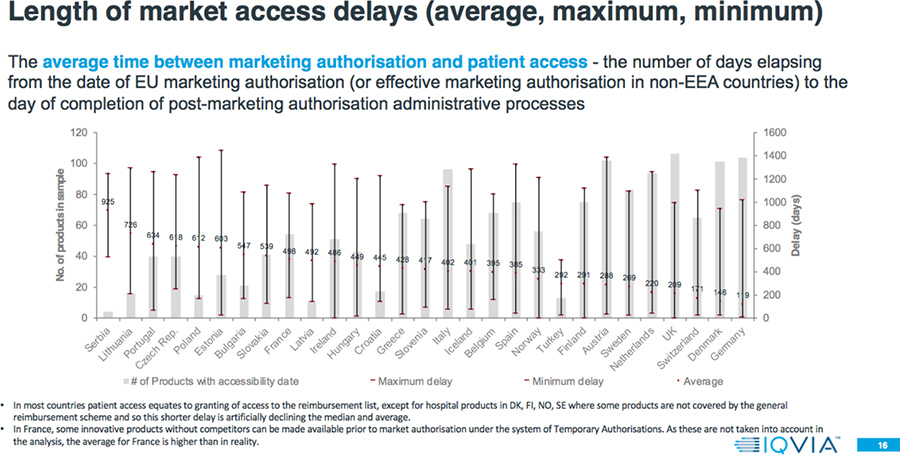
Results: Comparison to 2017 W.A.I.T. Indicator Study
Among countries overlapping both the 2017 and 2018 analyses, in the 2018 study 65 percent have a higher rate of availability, and 52 percent have a longer delay.
Conclusions and Next Steps
Examining disparities in the speed of access and availability of new medicines is the focus of EFPIA’s W.A.I.T. Indicator report. Everyone involved in healthcare–from patients to service providers, from researchers to clinicians, and from pharmaceutical companies to payers–wants to see patients across Europe get access to new treatment options. This report is designed to provide an accurate picture of the realities of access to medicines in Europe. It’s our hope that by focusing on the needs of patients and working with partners across healthcare we can find collaborative solutions to address the issues raised in this report.