Orion Innovation
Gan & Lee Pharmaceuticals USA Corp
BeiGene Corp
Apellis Corp
IQVIA
FTI Consulting
Phlexglobal
Apellis Corp
IQVIA
FTI Consulting
Phlexglobal
The Dilemma
Regulatory Information Management (RIM) has emerged as a fast-growing area to streamline and enhance the regulatory value chain with a set of capabilities built on process, data, technology, and organizational components. More recently, RIM data and system capabilities have evolved beyond merely supporting regulatory goals and objectives to providing integrated data flows with other functions (e.g., clinical, manufacturing, safety) across the value chain.
Accompanying this evolution has been a widely divergent understanding of RIM’s scope, nomenclature, terminology, processes, data requirements, etc., which has unfortunately resulted in incongruent processes, data structures, and terminology, thereby increasing complexity in building and operating regulatory information management capabilities.
The Solution
As part of its mission, the DIA RIM Working Group (“the group”) has proposed the RIM Reference Model V1.0 as a first step to address the process and data incongruency dilemma with a common framework for defining and managing data and information supporting regulatory activities. Just as the Trial Master File (TMF) model started out as a DIA-led initiative and has now become part of CDISC standards, there is opportunity for the RIM Reference Model to become a globally accepted standard in the future.
What is the RIM Reference Model?
The model was initially based on detailed analysis of processes around investigational and marketed regulatory activities and has been subsequently validated against the information needs of a CMC Change or Variation process. It has focused on drugs and biologics, with plans to extend to medical devices and combination products in the future. Unlike the Submission EDM and TMF Reference Models, which are based on artifacts such as documents and their metadata, the RIM Reference Model is about RIM processes, related objects, and their data and attributes. Therefore, the RIM Reference Model is process- and data-centric, while the EDM and TMF Reference are document/metadata-centric.
Benefits
Strategic benefits include:
- Improving speed to market
- RIM data feeds analytics, including forecasting, identification of constraints, trends, etc.
- Precision drives “right-first-time” results in terms of product registrations
- Supporting compliance
- Accurate data translates to better management of registrations and product change control
- Granular registration data drives “right product/right country” performance
- Aligning with data standards (including ISO’s Identification of Medicinal Products [IDMP] and EMA’s Substance, Product, Organization and Referential [SPOR]) increases accuracy
- Supporting capability development and interoperability
- Baseline data model facilitates faster implementation of technology capabilities
- Harmonization facilitates M&A integration
- Reducing costs
- Less time cleaning regulatory data creates operational efficiency
- Structured data facilitates automation (both robotic process automation [RPA] and artificial intelligence/machine learning [AI/ML]).
At the tactical level, the model helps regulatory and IT organizations better manage both regulatory data and integration with other functional systems such as ERP for product release, Quality Management Systems (QMS) and Labeling for product change control and variations, and pharmacovigilance for safety reporting.
The model enables process harmonization and interoperability since it provides best practices, common terminology in its objects, data concepts, relationships, and metadata. It provides a foundation for Master Data Management (MDM) that can be utilized across functions. By adopting some form of the model, companies can more easily exchange data across business functions and applications, and with business partners and health authorities.
The reference model also provides a baseline data and information model that addresses common needs of sponsors while enabling regulatory and other functional areas to have better visibility into regulatory activities and related information (e.g., identify potential extensions).
RIM Taxonomy
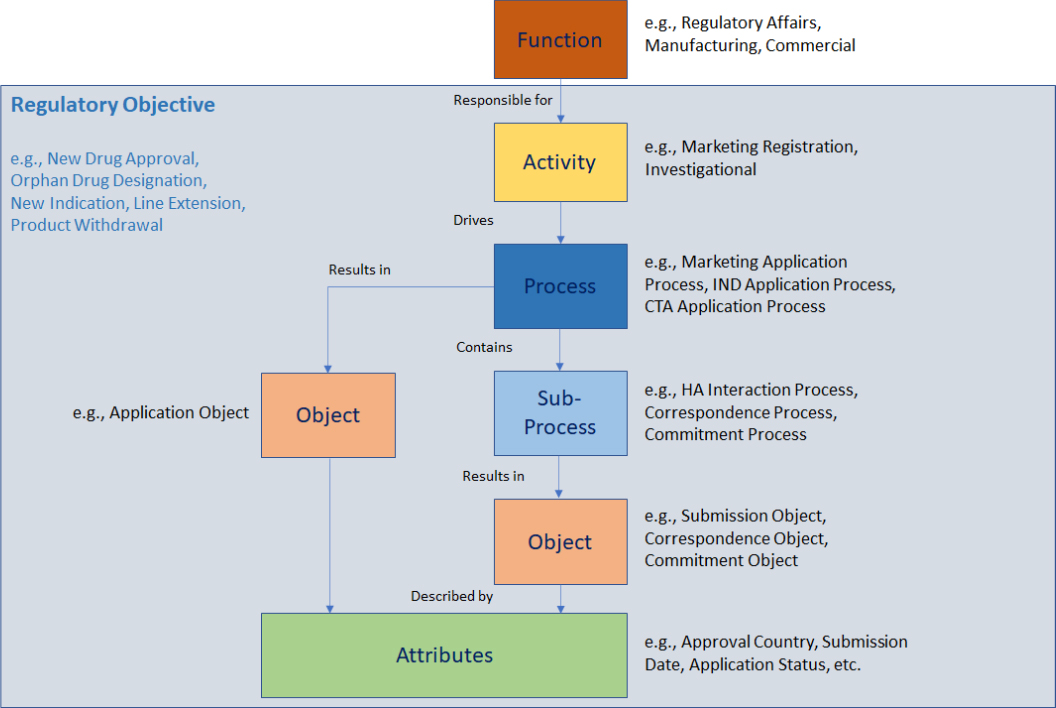
How to Use the Model
RIM Implementation: The model provides a baseline data model to drive configuration requirements for RIM implementations. Many companies, especially those early in their lifecycle, will have incomplete RIM data models. More mature companies may have RIM data sitting in disparate databases. The RIM Reference Model helps define a “to-be” state, enabling a rapid gap analysis. It also provides a starting point for a configuration model by allowing for definitive mapping of existing data to a generally accepted reference.
Master Data Management (MDM) initiatives: Regulatory affairs and its data are part of a much broader data and information ecosystem. For regulatory affairs to function effectively, they must integrate with processes and data controlled by other functions. The model can provide a guide to enterprise MDM organizations, helping them to understand data and governance requirements for the regulatory node of an enterprise data ecosystem and to enable cross-functional integration.
Mergers and Acquisitions (M&A): M&A activity abounds within the life sciences industry. The model provides a reference point for due diligence evaluations relating to RIM maturity and data quality. The model also supports data-mapping activity required for effective post-deal integration and regulatory data migration. During integration, it serves as a neutral ground that both the parent company and acquired organization can start from to achieve consensus around a common vision for the future state.
Call for Action
- We encourage vendors/system integrators to leverage the model in their product offerings and solution implementations; one method would be to use the reference model as the baseline for requirements definition and system configuration.
- We envision sponsors will pilot the model as part of their RIM initiatives; sponsors who have already implemented RIM may use the model to support M&A activities for mapping and migration purposes.
- We invite everyone to actively join the DIA RIM Working Group and participate in the evolution and development of the next version of the RIM Reference Model.

