What are the Needs, Challenges, and Opportunities?
ecision making in healthcare is a complex process that takes place along a continuum that moves from evidence generation to evidence review and deliberation, to decision making, and to communication of decisions made. In many low- and middle-income countries (LMICs), this process is often not systematic, deliberative, or transparent. This article highlights the links between Health Technology Assessment (HTA) and regulatory practices, including harmonization and collaborative efforts, in Latin America.
HTA Landscape in Latin America
Different countries are at different stages of developing HTA expertise, but the importance of HTA is increasing across Latin America. Some countries have little or no awareness of HTA as a policy solution to improve efficiency, transparency, and accountability of resource allocation. A few nations (e.g., Brazil and Colombia) have established mature HTA systems that inform value-based national healthcare. Others have already established (e.g., Argentina and Mexico) or are close to establishing or broadening the mandates (e.g., Chile and Peru) of their national HTA agencies.
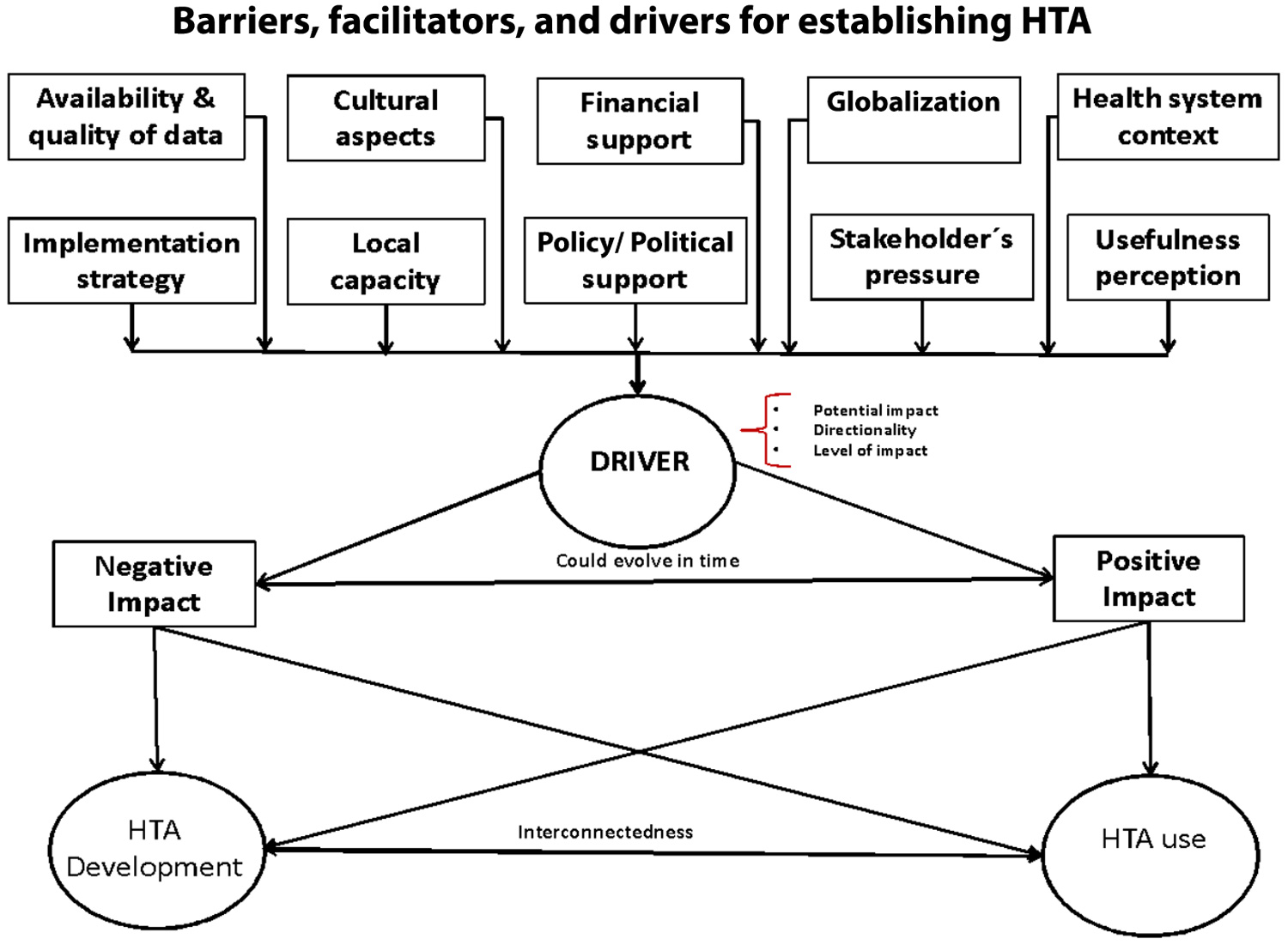
Barriers to establishing HTA include lack of financial support, policy/political support, local capacity, data quality, and implementation strategy (see Figure 1). For example, in Colombia, major policy changes over the last decade have boosted HTA development; local policy support from governmental institutions has resulted in funding for capacity building at HTAs within the Institute of Health Technology Assessment (IETS).
Collaboration and Harmonization in Latin America HTA
In 2020, an international joint task group co-led by the International Network of Agencies for Health Technology Assessment (INAHTA) and Health Technology Assessment International (HTAi) published a new and internationally accepted definition of HTA. However, some authorities, regulators, payers, and stakeholders in Latin America continue to use outdated definitions of HTA to inform their decision-making. Therefore, development partners within the region need to advance and evolve the definition and application of HTA to reflect current international standards and best practices.
There have also been some global efforts to advance the institutionalization or establishment of HTA mechanisms in many LMICs. One organization has developed a roadmap, which outlines opportunities to align the processes of national regulators and HTA mechanisms, for establishing HTA in LMICs.
Regulatory-HTA Alignment
HTA agencies may have opportunities to align with regulators and leverage regulatory information, for example as early research transitions into product development, or by using information already submitted in the regulatory dossier for purposes of making HTA decisions. Reliance on cross-border HTAs is not common practice in the region, but there may be an opportunity to explore a “core” regional model like that of EUnetHTA in the EU.

