Around the Globe
Asia-Pacific Digital Health and Data Consortium
Roche Pharmaceuticals
Merck Sharpe and Dohme
Johnson & Johnson
Takeda
group of industry partners have formed the APAC Digital Health and Data Consortium (ADDC) to serve as a platform for collaboration between industry and other organizations such as policy think tanks and regulators to shape digital health data frameworks in the APAC (Asia-Pacific) region.
In addition, there has been rapid digitalization of healthcare, resulting in new sources of data emerging across the world. Consequently, health data is increasingly being generated outside clinical trials, which is also referred to as real-world data (RWD). These RWD sources encompass a wide array of inputs ranging from electronic health records (EHRs), insurance claims and administrative databases, and patient registries, to wearables and in-home monitoring, health survey data, and beyond. Finally, the COVID-19 pandemic accelerated our knowledge of how digital health and data from these digital health tools and digitalization can be used to monitor and address population healthcare needs.
For efficient (or optimal) use of digital healthcare, a key driver is the creation of frameworks for digital health technologies and solutions. Therefore, it is encouraging to see organizations such as APACMed create materials that may help drive the creation of a fit-for-purpose regulatory framework for digital health across the APAC region for device regulators. However, we also need to ensure that regulatory frameworks are in place that address healthcare data policies. This will allow us to utilize digitalized data sources (such as RWD) as well as data from digital health tools for downstream uses (e.g., using digital measures to develop novel endpoints for clinical trials, etc.).
With these new sources of data, it is important to draw a distinction between digitalized data (e.g., electronic health records) and data from digital health tools. Generally, digitalized data is considered “static” (i.e., there is usually a time lag between data collection and data availability for analysis), whereas data from DHTs can be considered “continuous,” because it can be available in almost real time. This continuous stream of data may provide the opportunity to demonstrate real-time performance data which can greatly enhance our ability to address healthcare needs quickly and allow us to measure variables (e.g., via sensors) that we were not able to measure before. As with the use of any type of data by regulators, we need to ensure that implementation of such regulatory frameworks will enable utilization of these types of data.
However, uptake of digital health and implementation of healthcare data policies differ significantly across ASEAN countries. The adoption of digital health innovation and collaborations throughout Southeast Asia and the wider Asia-Pacific region seems to vary. Other issues include the lack of streamlined regional healthcare data policies and the rapidly changing healthcare data environment, both of which hamper drafting policies for interoperability in a timely manner. The ADDC has been formed to address this gap.
Vision, Mission, and Establishment of ADDC
ADDC has outlined specific near-term and midterm objectives with this vision in mind:
- To establish ADDC as the leading industry consortium playing a key role in shaping use of digital health data within this region
- To help shape or co-create regulatory frameworks as needed to enable use of digital health data by regulators in this region and beyond
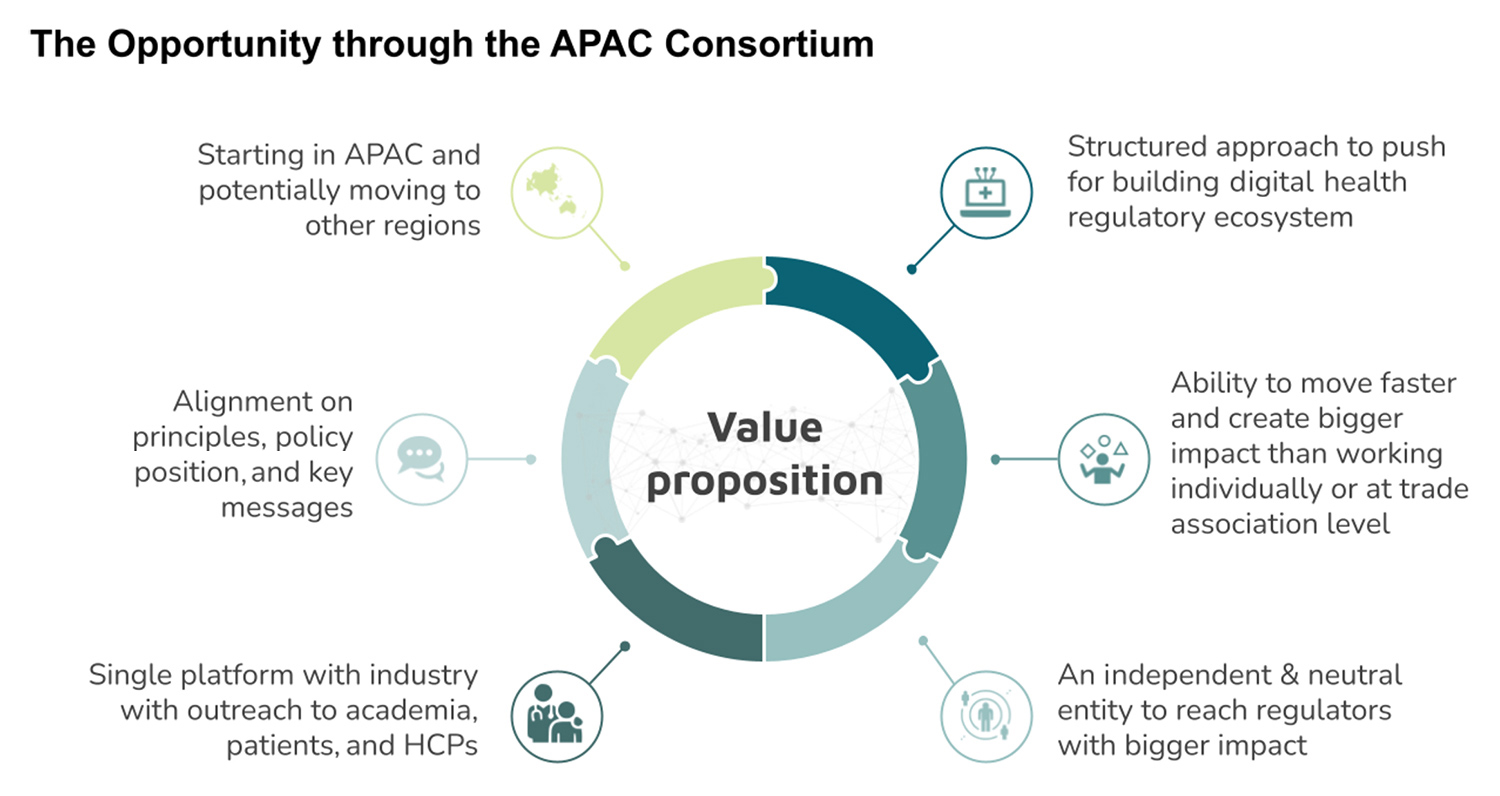
Created in 2020, the consortium currently consists of four industry partners (Johnson and Johnson, Takeda, Merck Sharpe and Dohme, and Roche Pharmaceuticals) that have a significant footprint in this region. We envisage working with multiple stakeholders such as policy think tanks, academia, patients, healthcare professionals (HCPs), and regulators to drive toward our objectives. The figure below highlights numerous opportunities available through the creation of such a consortium.
Achievements of ADDC: 2020-2021

Plan for 2022-2023 and Call for Additional Partners
Conclusion
- Shaping the regulatory environment to enable the use of DHTs and tools, and digitally derived data, for product development and regulatory decision making; and
- Fostering efficient and patient-centric drug development through use of digital healthcare data.
Acknowledgements: The authors thank the following additional contributors to this project: Sannie Chong (Roche Pharmaceuticals) and MayLi Woon (Sanofi).

