University of California, San Francisco
University of Pennsylvania
University of Texas MD Anderson Cancer Center
University of California, San Francisco
University of Minnesota
University of California, San Francisco
University of California, San Francisco
University of California, San Francisco
ince opening in 2010, 22 agents or combinations of agents have entered I-SPY 2–a long-standing, adaptive platform trial of neoadjuvant therapy for patients with stage 2-3 breast cancer with high risk for early recurrence. Twelve agents have completed accrual and seven have “graduated” in at least one tumor subtype (indicating an 85% probability that the agent will be successful in a confirmatory phase 3 trial).
Key Advancements of the I-SPY 2 Platform Trial:
- pCR is an important prognostic marker for individual patients with molecularly high risk disease, regardless of treatment or subtype received.
- We developed an MRI-based “predicted Residual Cancer Burden” (pre-RCB) strategy to predict pCR after each regimen, but before surgery, with high specificity and moderate sensitivity and are testing it prospectively. Furthermore, ctDNA is being developed as an adjunct to improve residual disease prediction and outcome.
- Improved classification of tumors improves predicted pCR rates with optimal agent assignment for the molecularly high risk population.
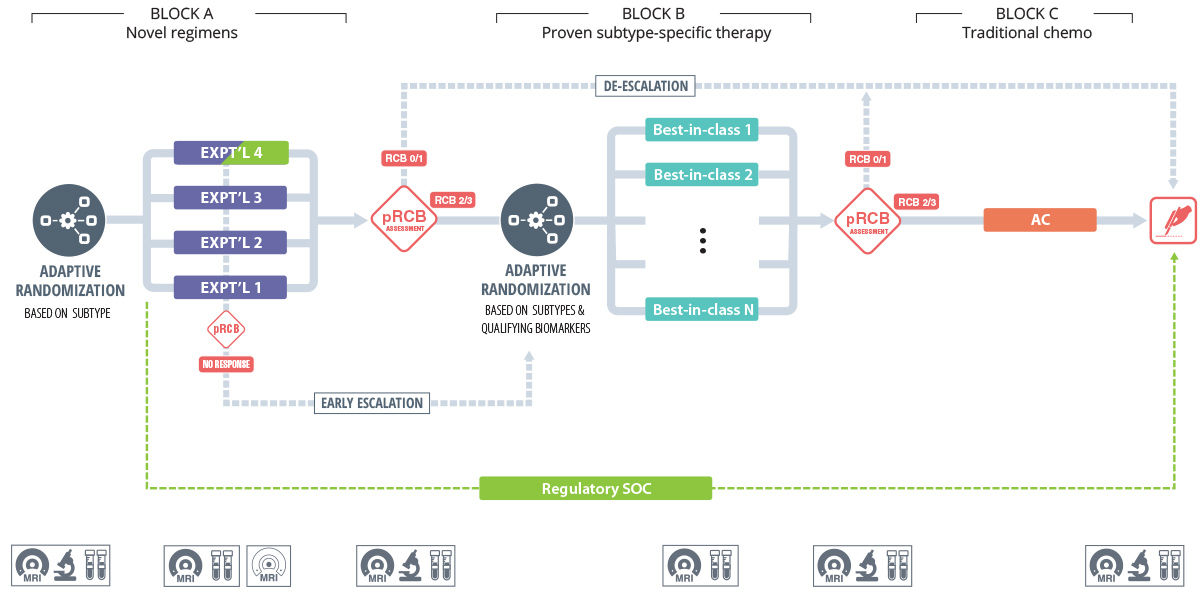
The I-SPY 2.2 Design
I-SPY 2 is evolving, in part, due to the success in identifying treatments that improve subtype-specific response. New opportunities to improve care for women also stem first from the prognostic relationship of pathological complete response (pCR) to event-free survival (EFS) and distant recurrence–free survival (DRFS), and second from the additional predictive value in quantitative residual cancer burden scoring and the finding that additional chemotherapy after achieving pCR does not improve survival. Third is the emergence of exciting new therapeutics, and fourth is that clinicians would like to avoid giving doxorubicin-containing regimens, if possible. We have been working with the FDA for a number of years on the optimal strategy for “I-SPY 2.2.” An NCI-funded program project grant has enabled us to focus on developing and testing more patient-centric trial models.
We (the clinician and advocate communities) are advancing a more personalized strategy to safely allow patients with excellent response to receive less treatment (and therefore less toxicity), while those with suboptimal response may receive additional therapies.
Our design strategy forms a framework for advancing personalized medicine, where novel treatments are assigned based on biology and response, utilizing direct measures to monitor response during treatment (e.g., MRI volume change) and after treatment (residual disease at surgery). The goal is to get each individual to the optimal early endpoint, which at this time is pCR/low RCB. Imaging has played an important role in enabling us to make these changes.
I-SPY 2.2 will enable testing of emerging targeted agents and combinations of biologics and allow those agents to either be combined with less toxic chemotherapeutic regimens or to replace cytotoxic chemotherapy entirely when less toxic agents are sufficiently effective. Importantly, when these are not successful, the next line of therapy will be the optimal regimen for their subtype using a taxane combination.
Our strategy to optimize response assesses the impact of each therapeutic regimen based on change in MRI volume as well as changes in other biomarkers, including ctDNA (Figure 1).
- Block A: Test the most promising novel agents without standard chemotherapy.
- Targeted de-escalation: Patients who are predicted to have a complete response after Block A will proceed to surgical excision.
- Targeted early escalation: Patients with a poor imaging response at 3 weeks will repeat imaging at six weeks and proceed to “Block B” if response is not identified.
- Block B: Patients will proceed to the optimal treatment combinations (best in class for each subtype, based on results from I-SPY 2 and other practice changing trials).
- Block C: Patients with persistent disease after Block B will proceed to AC (adriamycin/cyclophosphamide) those with predicted complete response will proceed to definitive surgical resection.
This approach also allows us to evaluate and optimize the sequence of therapy–minimizing toxicity and maximizing benefit–all while providing a regulatory framework and rapid path to approval for the most promising agents.
Toxicity reduction is a central objective of the updated design. I-SPY 2 has enabled the identification of agents, mostly in combination with taxanes, that have substantially increased the chance of pCR. However, continuing to improve outcomes does not mean simply adding more drugs. This approach increases toxicity (and cost) in many patients who would do just as well with a less extensive treatment. I-SPY’s goal is to identify new rational therapies for patients who need them, while also identifying those who can be cured with less therapy, toxicity, and cost. In particular, we aim to start treatment with emerging targeted agents in a subtype-specific manner, forgoing standard chemotherapeutic agents if they are not needed. This approach emphasizes toxicity reduction as a clinical endpoint – one that is of clear importance to patients, their physicians, and regulators.
The trial will continue to leverage the significant advantages of the neoadjuvant setting to implement targeted, response-based escalation or de-escalation of therapy, carried out in a sequential manner, to achieve early (pCR) and long term (DRFS) success for patients.
Our overarching goal is to identify treatment strategies that yield sufficiently high rates of short-term response to result in a three-year DRFS of more than 90% for each tumor subtype.
Integrating a Seamless Transition to Phase 3
With the I-SPY 2.2 design, we will extend the efficiency of the original design to enable seamless phase 3 confirmation. As the I-SPY network of clinical sites has grown (there will be 28 sites by the end of Q2 2021), so too has the opportunity to further improve the efficiency of the model. I-SPY 2.2 will leverage both the proven I-SPY network and the data collected in phase 2 to seamlessly transition successful, novel agents to phase 3 (Figure 1).
An additional focus of I-SPY is the identification and validation of biomarkers to improve biological targeting of therapy, incorporating prospective evaluation of qualifying biomarkers (conducted in CLIA [Clinical Laboratory Improvement Amendments] laboratories and analyzed independently). Clinical and outcomes data for all patients are supplemented by full transcriptome array tumor profiling, phospho-protein arrays, immune multiplex assays, circulating tumor DNA (ctDNA), and other targeted interrogations. Over the past decade, we have learned how to increase the overall pCR rate to known agents by improving tumor classification. For example, a third of hormone receptor-positive tumors are Mammaprint® ultrahigh (corresponding to the basal phenotype) that have a robust response to added PD1 and PDL1-inhibitors, and triple-positive luminal B tumors are less responsive to Her2-targeted therapy.
We are on track to transition to the phase 2 (signal finding phase) of I-SPY 2.2 by late 2021, and are choosing agents and combinations now. The key milestones for the full transition include refining clinical workflows for de-escalation and operationalizing early escalation, and implementing enhanced tumor classification for adaptive randomization.
We have also implemented a dynamic control (enabling the best treatments, including control, to be assigned adaptively) and will institute a graduation threshold based on the performance of the entire treatment sequence, as well as on the performance in Block A and the ability to decrease the total amount of therapy for some fraction of the population. Once we have identified agents that add to pCR and, in particular, reduce the total burden of treatment, they will seamlessly transition to a confirmatory phase 3. The new agent combination alone, or in sequence with optimal agents by subtype, will be compared to the subtype-specific regulatory standard-of-care (Figure 1). Three-year survival endpoints of more than 92% for those with pCR will be the confirmatory endpoint for approval. A type C meeting with the FDA is being set for review of the seamless phase 2/3 component of the trial in late 2021. The goal is to find sequences of agents that maximize every patient’s chance of cure, and to get 90% of patients to complete response using our biologically driven, rapid-learning trial platform.

