Around the Globe
Bill & Melinda Gates Foundation
OVID-19 has brought us all into unchartered waters. Many African countries, following the advice of the World Health Organization (WHO), have implemented detection and control measures including the lockdowns of entire countries. According to the WHO and other public health experts, health systems in Africa are predicted to become overwhelmed by the COVID-19 burden if aggressive measures to prevent the spread of coronavirus are not implemented. As of May 31, 2020, the cumulative COVID-19 cases in Africa were over 100,000 (Source: WHO Coronavirus disease (COVID-2019) situation reports).
On May 8, AVAREF endorsed an emergency expedited clinical trial review guidance for its joint and assisted review procedure. The guidance recommends a submission-to-decision timeline of ten working days for already licensed products with an existing indication (being repurposed for COVID-19), and fifteen working days for a novel product. These are very tight timelines even without the current lockdown situation and will require significant changes to the processes of reviews of clinical trial applications (CTAs) by ethics committees and regulatory authorities in individual countries to meet. AVAREF is obtaining country-by-country information on how sponsors can submit protocols in different jurisdictions, and how countries will be supported to meet these ambitious but critical timelines. The guidance has been published on the AVAREF website hosted by the WHO Regional Office for Africa Server.
On March 18, the WHO Director General invited countries to participate in Solidarity Trials. By April 8 April, more than 100 countries had expressed interest to participate. On April 24, WHO launched the Accelerator for R&D, which is aimed at accelerating development and availability of new COVID-19 tools; accelerating equitable global access to safe, quality, effective, and affordable COVID-19 diagnostics, therapeutics and vaccines; and to ensuring that the fight against COVID-19 leaves no one behind.
Some African countries have already reviewed and authorized clinical trials of therapeutics as part of this collaborative effort, while others are about to receive submissions. The AVAREF guide will serve a useful purpose in supporting countries to meet competitive timelines for reviews of clinical trial applications.
AVAREF Expedited Emergency Joint Review Procedure
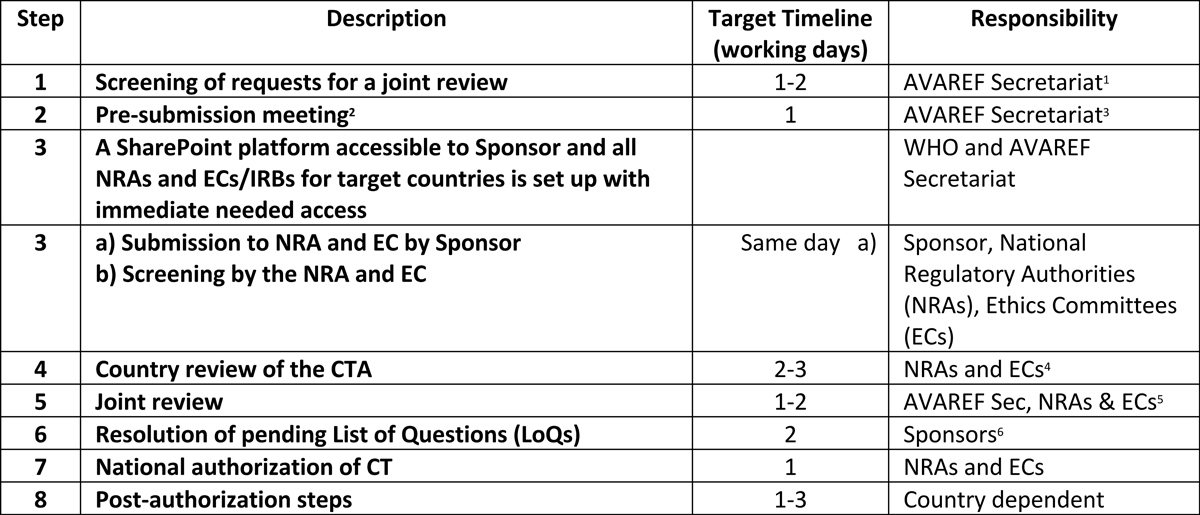
2: Pre-submission meeting conducted virtually, resulting in agreement on all aspects of actual submission.
3: Key NRA and EC decision makers should attend pre-submission meetings and be involved in the entire expedited review process to ensure agreement on expectations, roles, and responsibilities.
4: Accomplished in the most efficient means possible as agreed in the pre-submission meeting.
5: Key NRA and EC decision makers should attend virtual review, because agreements and decisions on timelines must be made during the technical review.
6: Channels for submission of LoQs are agreed upon during the joint review meeting.

