Yi Liu
Medical Director
dMed Biopharmaceutical
edical Affairs covers a wide range of responsibilities in the pharmaceutical industry, but none is more important than its bridging of patients, policy makers, pharmacists, and physicians. The medical affairs agenda at the DIA China Annual Meeting 2019 shared forward-looking insights on medical ethics, real world evidence, and medical affairs’ evolving role in local biotechnology projects and organizations launching new drugs in China.
Key Takeaways
- The crux of the ethical issue in interactions between the pharmaceutical industry and healthcare professionals is the complex relationship among scientific innovation, patient care, and commercial benefit.
- The difference in the effect estimate between observational studies of real world data and data generated by randomized clinical trials is often not due to the difference in their study methodologies per se, but to the level of heterogeneity of the studies.
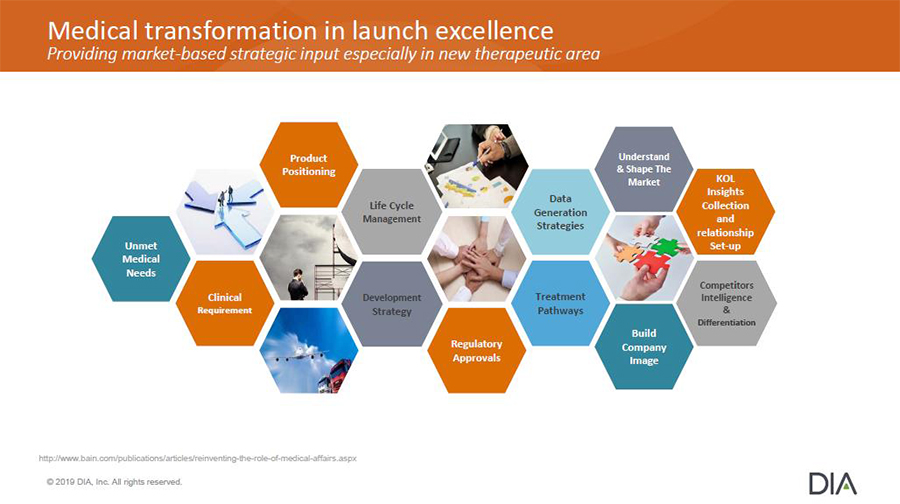
- A patient-centric and innovative new product launch plan is based on a medical strategy built upon three key pillars: the patient’s medical story or journey, evidence generation, and innovative medical education.
Medical Ethics
Liming Wang, a renowned popular science writer and professor in biology, cited gene edited babies and related experimental scandals as case studies for the need to raise awareness of medical ethics management and for constructing a proper ethics oversight system, as are cases of “ethics dumping,” when developed-world scientists conduct unethical or questionable research in low-income countries.
The crux of the ethical issues in interactions between the pharmaceutical industry and healthcare professionals is the complex relationship among scientific innovation, patient care, and commercial benefit.
Utilization and Challenges of Real World Data
The EMPRISE (Empagliflozin Comparative Effectiveness and Safety) Study was presented as a Real World Evidence Case Study. Designed to generate generalizable information on the effectiveness and safety of empagliflozin from a broader patient population when treated in routine clinical care, EMPRISE also answered questions, such as the drug’s impact on healthcare resource utilization and the cost of a real world study, that the original pivotal clinical trials were not designed to address. Results from real world studies with study designs that minimize confounding factors and potential biases, while taking precautions with the interpretation of results, could complement randomized clinical trials; perhaps these findings can be translated into routine clinical practice.
Real world data analyses of patients receiving routine care have provided findings similar to those found in randomized clinical trials, which may support the effectiveness of supplemental indications for approved medications. The difference in the effect estimate between observational studies of real world data and data generated by randomized clinical trials is often not due to the difference in their study methodologies per se, but in the level of heterogeneity of the studies.
Medical Affairs in Domestic Pharmaceutical Companies
The goal of product lifecycle management—to maximize the value of an individual product—requires cross-functional collaboration. Medical Affairs plays a critical role in such tasks as new formulations, new administration routes, revised dosages, new indications, and patient population expansion. This applies to new product development to developing the robust clinical evidence package to product launch to maintaining a mature product portfolio.