Around The Globe
African Medicines Agency Establishment Treaty Adopted by African Ministers of Health
Leveraging Existing Regulatory Assets to Improve Access to Essential Medicines
Margareth Ndomondo Sigonda
Head of Health Programmes,
NEPAD Agency
Margaret Agama-Anyetei
Head of Division Health, Population and Nutrition, Department of Social Affairs,
African Union Commission (AUC)
Gugu Mahlangu
Director General,
Medicines Control Authority of Zimbabwe (MCAZ)
Hlazo Mkandawire
Programme Officer, Communication,
NEPAD Agency
n 19 May 2018, the African Ministers of Health unanimously adopted the draft Treaty for the establishment of a single continental body for the regulation of medicines and medical products, the African Medicines Agency (AMA). Once finalized, the Treaty will be presented to African Union (AU) Heads of State and Government for endorsement in January 2019.
The Ministers of Health convened on the sidelines of the 71st Session of the World Health Assembly (WHA) in Geneva, Switzerland as a working group of the Specialized Technical Committee on Health, Population and Drug Control (STC-HPDC) of the AU. The establishment of AMA will contribute to ensuring that the health of the African people is protected from threats posed by Sub-Standard and Falsified (SF) medicines, medical products and technologies. AMA will also work with the AU to promote the growth of the local pharmaceutical industry on the Continent through its Policy Framework, the Pharmaceutical Manufacturing Plan for Africa (PMPA). PMPA has a clear vision on the development of the pharmaceutical industry in Africa.
The journey to preparing the AMA Treaty to a level considered ready for adoption by African Ministers of Health has not been a walk in the park. It has involved meticulous planning led by a Task Team and coordinated by a joint secretariat made up of the Africa Union Commission (AUC), the New Partnership for Africa’s Development (NEPAD) Agency, and the World Health Organization (WHO). Extensive consultations on AMA took place across the African continent. In 2017, three continental consultations with legal and regulatory experts were conducted to review the draft AMA Treaty in Johannesburg, South Africa; Addis Ababa, Ethiopia; and Tunis, Tunisia. Following these consultations, a draft AMA Treaty was developed and presented to the African Ministers of Health in 2017 on the margins of the 67th session of the Regional Africa Committee (RAC) of the WHO in Victoria Falls, Zimbabwe. It was at this meeting that the Ministers requested more time to review the AMA Treaty and make further consultations in their respective countries for ownership.
African Ministers of Health have remained firm in their resolve to set up a single continental medicines regulatory agency from the time they adopted the Luanda Commitment in 2014. The Luanda Commitment paved the way for establishment of the AMA by mandating the AUC to establish a Task Team to oversee the establishment of AMA, define its scope, and its financial and policy implications with technical support from the WHO. Hence, the resulting AMA Treaty demonstrates the strong collaboration between AUC, the NEPAD Agency, and WHO. There is a need for support from AU Member States in order to establish an AMA that will be a strong and legitimate institution with a buy-in from all countries. The adoption of the AMA Treaty by the African Ministers of Health is a clear demonstration of their commitment to strengthening medicines regulation in Africa.
How Will AMA Benefit the African People?
Based on the recommendations of the Luanda Commitment, the roadmap for the establishment of AMA was endorsed by the African Union Executive Council decision in 2015. African Ministers of Health adopted the AMA Treaty because they are convinced that AMA will provide an enabling regulatory environment for research and development as well as innovation and local production of pharmaceuticals, optimizing competitiveness and expanding markets. This will make medical products easily accessible and affordable in the long term. The decision of the African Ministers of Health in Geneva is motivated by the mission of the AU PMPA Framework to facilitate the development of a competitive pharmaceutical industry in Africa to ensure self-reliance. The African pharmaceutical industry is expected to grow and will be worth between US $40-65 billion by 2020. Through the PMPA Framework and the African Ministers of Health decision to adopt the AMA Treaty, future establishment of AMA will ensure that African people are able to harness this expected growth in the African pharmaceutical industry to create jobs for the youths and ignite social and economic development on the continent.
AMA is predestined to becoming an African-driven, independent, strong, and strategic institution for strengthening medical products’ regulatory systems on the continent and pulling together human expertise and financial resources to effectively manage the prevalence of Substandard and Falsified (SF) medical products, and address the burden of access to essential medicines. Currently, prevalence of SF medical products is rampant due to weak regulatory systems. Furthermore, half of the population in the most remote rural parts of the continent have no access to lifesaving, life-prolonging medicines. This poses a series of risks to public health.
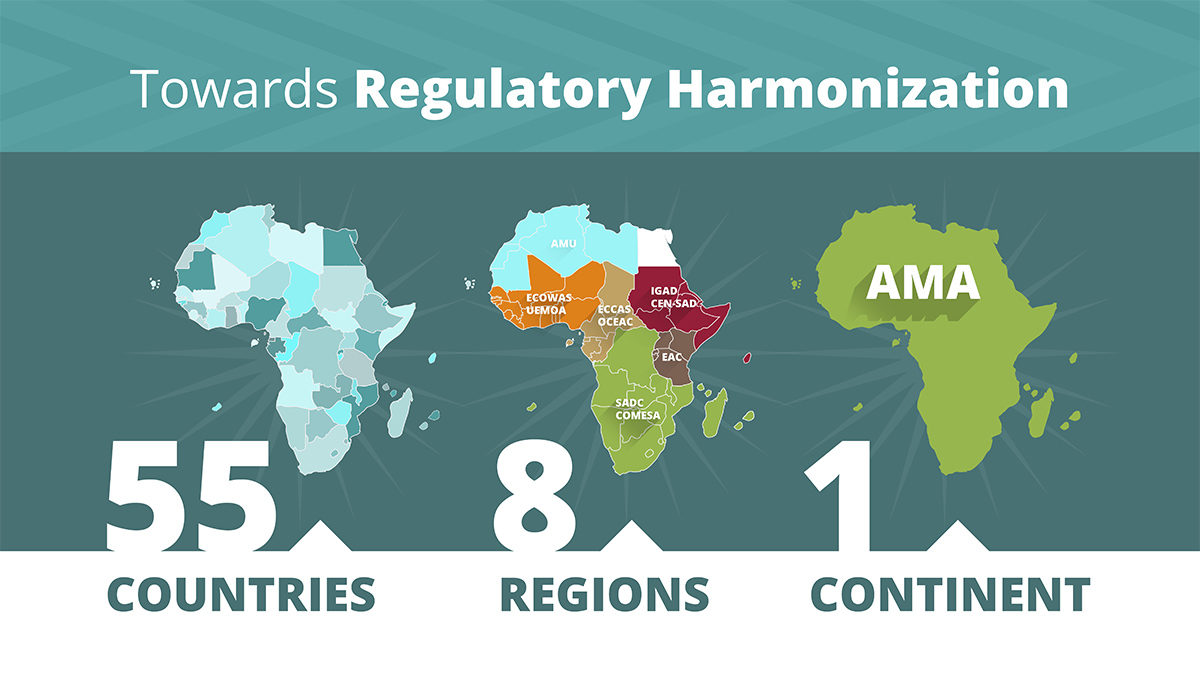
Regional Harmonisation Initiatives in Africa
Economic Community of West African States (ECOWAS)
- The ECOWAS region will hold its first joint dossier assessment in Dakar Senegal during the week of 25 June.
- ECOWAS comprises 15 countries: Benin, Burkina Faso, Cape Verde, Cote d’Ivoire, Gambia, Ghana, Guinea, Guinea Bissau, Liberia, Mali, Niger, Nigeria, Togo, Senegal, and Sierra Leone.
- During the joint assessment session, the ECOWAS Medicines Evaluation Expert Working Group (EWG) will be joined by experts from the European Medicines Agency (EMA). The EMA will provide training and share experiences with their West Africa colleagues on dossier assessment. The EMA will also discuss their EMA Article 58 procedure and explore how both parties can collaborate through this important procedure.
Southern African Development Community (SADC)
- The countries of the SADC region will hold their 2nd joint assessment meeting for 2018 in Lusaka, Zambia. To date, over 200 products have gone through the SADC joint assessment process (better known as the ZAZIBONA process), with 140 assessments completed and the rest at various stages of assessment.
- During this meeting, experts from the EMA will offer training on assessment of biologicals and biosimilars.
East African Community Medicines Regulatory Harmonisation (EAC MHR)
- A recent council of health ministers meeting held in Kigali, Rwanda, in May 2018 approved a new 5-year plan (2018-2022) for the EAC Medicines Regulatory Harmonisation as well as a cooperation framework agreement for the national medicines regulators. This plan and the accompanying cooperation framework seek to address the challenges identified in the previous phase through (i) strengthening of the technical coordination of the joint activities, (ii) building a binding mechanism for regional recommendations, and (iii) creating a sustainability pathway through greater ownership and financing of work-sharing by the member states. The plan is expected to come into effect in July 2018.
The ultimate goal of establishing AMA is to have very strong national regulatory systems, with excellent technical backup at regional and continental levels in Africa. These sentiments are shared by the Chair of the AMA Task Team and Director General at Medicines Control Authority of Zimbabwe (MCAZ), Gugu N. Mahlangu. She states that it is increasingly becoming evident that no single country has enough resources and capability to efficiently and effectively regulate the whole supply chain system alone in this globalised world. Thus, AMA occupies a distinct position to leverage various regulatory assets and capabilities to improve access to essential medicines and health technologies that are safe, effective, affordable, and of good quality.
Harmonization efforts have already proved successful at a regional level in the East African Community (EAC) and the Southern African Development Community (SADC) through the ZAZIBONA approach (a collaboration between national medicines regulatory authorities in Botswana, Namibia, Zambia, and Zimbabwe). Other regions have also rolled out regional medicines regulatory harmonization initiatives at various stages of implementation: the Economic Community of West African States (ECOWAS) through collaborative efforts between the West African Health Organization (WAHO) and the West African Economic and Monetary Union (WAEMU), the Inter-Governmental Authority on Development (IGAD), and the Organization of Coordination for the Fight against Endemic Diseases (OCEAC) under the Economic and Monetary Community of Central Africa (CEMAC).
The success of these regional medicines regulatory harmonization initiatives in Africa serves as evidence of the increasing need for harmonization and act as a precursor to the benefits the continent will reap from the establishment of AMA. AMA will also ensure that the various development partners investing in these regional initiatives as well as country-level activities get value for their resources. This can be achieved through effective coordination of activities and by safeguarding the medicines regulatory space in Africa from duplication. Simply put, AMA will add personality and a continental structure to the current medicines regulatory harmonization work in Africa.
This added value of AMA cannot be over-emphasized. Speaking on behalf of the AUC Commissioner for Social Affairs, H.E. Amira Elfadil, the Permanent Observer of the AU to the United Nations (UN) Office at Geneva, Ambassador Ajay Kumar Bramdeo said that AMA is a key element of the architecture for harmonisation of continental, institutional, scientific, and regulatory resources to improve access to safe, efficacious, and quality medicines. Coordination of scientific expertise, a network of quality control of laboratory services, and capacity building of African regulators across different regulatory functions and product categories forms an integral part of AMA, among other functions. This continental approach is not a new undertaking as it has been done before and proved to be a success in Europe with the European Medicines Agency (EMA).
AMA is taking a strategic step-wise approach by leveraging existing initiatives, such as the African Medicines Regulatory Harmonization (AMRH) and African Vaccines Regulatory Forum (AVAREF), as building blocks to addressing regulatory challenges faced by African countries in ensuring effective coordination, scaling up activities and inculcating sustainability aspects.
The proposed establishment of AMA comes at the right time following the decision by the 44 African leaders to sign the agreement establishing a Continental Free Trade Area (CFTA) on 21 March 2018 in Kigali, Rwanda. The creation of the free trade area is billed as the world’s largest in terms of participating countries and is seen by many as the launch platform for the continent’s economic development. It is testimony to how Africa is becoming more integrated, and it presents an opportunity on how to advance the pharmaceutical industry through the CFTA framework. Historically, Africa has lagged behind in the area of medicines regulation, which compromises the continent’s ability to provide affordable access to lifesaving, essential medicines in the global quest to advancing the right to health for all and to achieving Universal HealthCare (UHC). This challenge is multi-faceted and cannot be resolved using a singular approach. It requires a methodical and in-depth analysis of the public health system on the one hand, and formulating sustainable mechanisms deliberately designed to chip-away at the challenge from different vantage points on the other hand. It is time for change that will ensure “Africa people have a high standard of living and quality of life, sound health, and well-being” as embodied in Aspiration One and Goal One of the African Union (AU) Agenda 2063 – a roadmap for the continent’s development agenda guided by the principle of delivering the #AfricaWeWant (Africa driving its own development agenda).
Conclusion
With support from the AU Member States, the establishment of AMA will come to fruition. What remains is for African Heads of State to endorse the transformation of Africa’s pharmaceutical industry by signing the AMA Treaty and promote the local production of pharmaceuticals on the continent. Once the required minimum number of countries ratify the Treaty, AMA will be established. Once established, AMA will not only serve to roll back the prevalence of SF medical products and technologies but also ensure easy and affordable access to essential medicines on the continent. AMA is the cornerstone of establishing a sustainable public health system in Africa.

