Sybil N.A. Ossei Agyeman Yeboah
West African Health Organization, Burkina Faso
Bill and Melinda Gates Foundation
rom 2018 to April 2022, the regional ECOWAS Joint Assessment Procedure (JAP) under the West Africa Medicines Regulatory Harmonization (WA-MRH) Initiative received twenty-two (22) dossier submissions from manufacturers within and outside the ECOWAS region, a relatively lower number than anticipated.
The West Africa Medicines Regulatory Harmonization (WA-MRH) was established in 2017 to improve the availability of medicines and vaccines of good quality, safety, and efficacy in the Economic Community of West African States (ECOWAS) pharmaceutical market, by strengthening and harmonizing drug regulatory systems to bring efficiency and transparency in the registration of medicines and vaccines.
A questionnaire was developed on Microsoft Form and distributed online to over 250 manufacturers located in West Africa and beyond. The questionnaire was developed by the WA-MRH Secretariat in consultation with project partners (including the World Health Organization, Swissmedic, and Bill & Melinda Gates Foundation) and addressed the following criteria:
- The manufacturer’s location
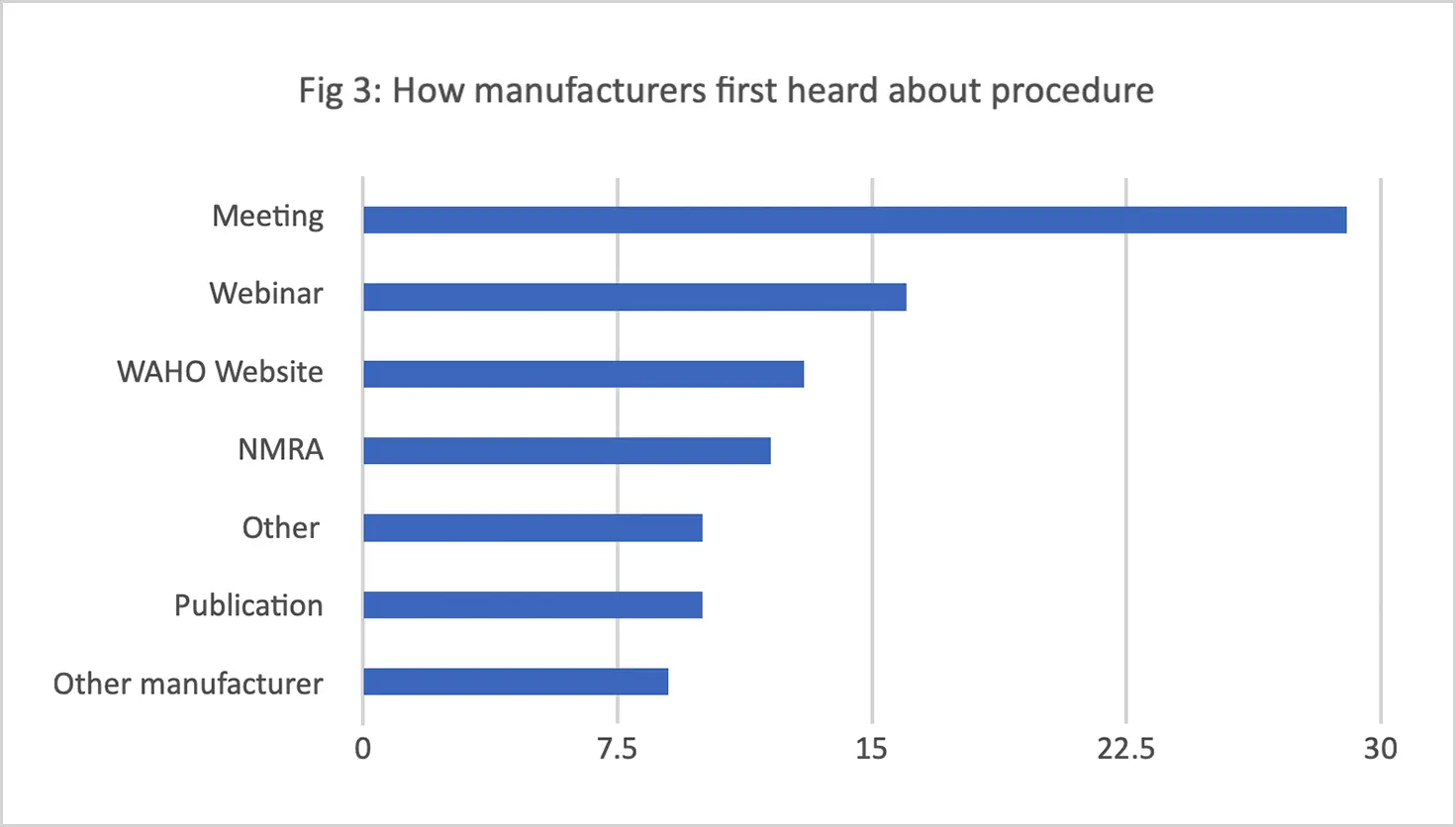
- Whether the manufacturer is aware of the process
- Whether the manufacturer subsequently participates in a regional or international process like the ECOWAS process
- The benefits expected by the manufacturer using the ECOWAS process
- The difficulties encountered by the manufacturer in using the ECOWAS process
- Suggestions to improve the process.
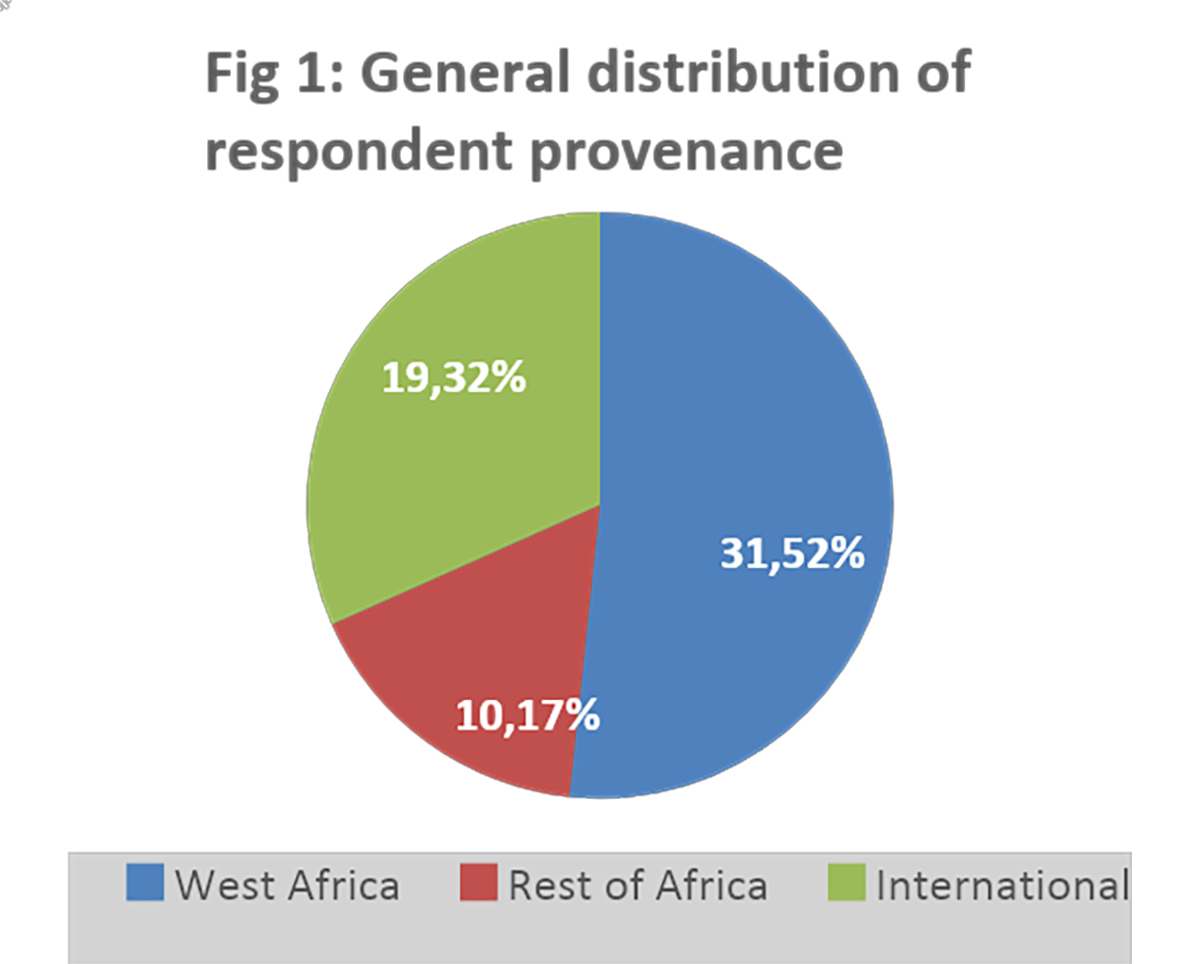
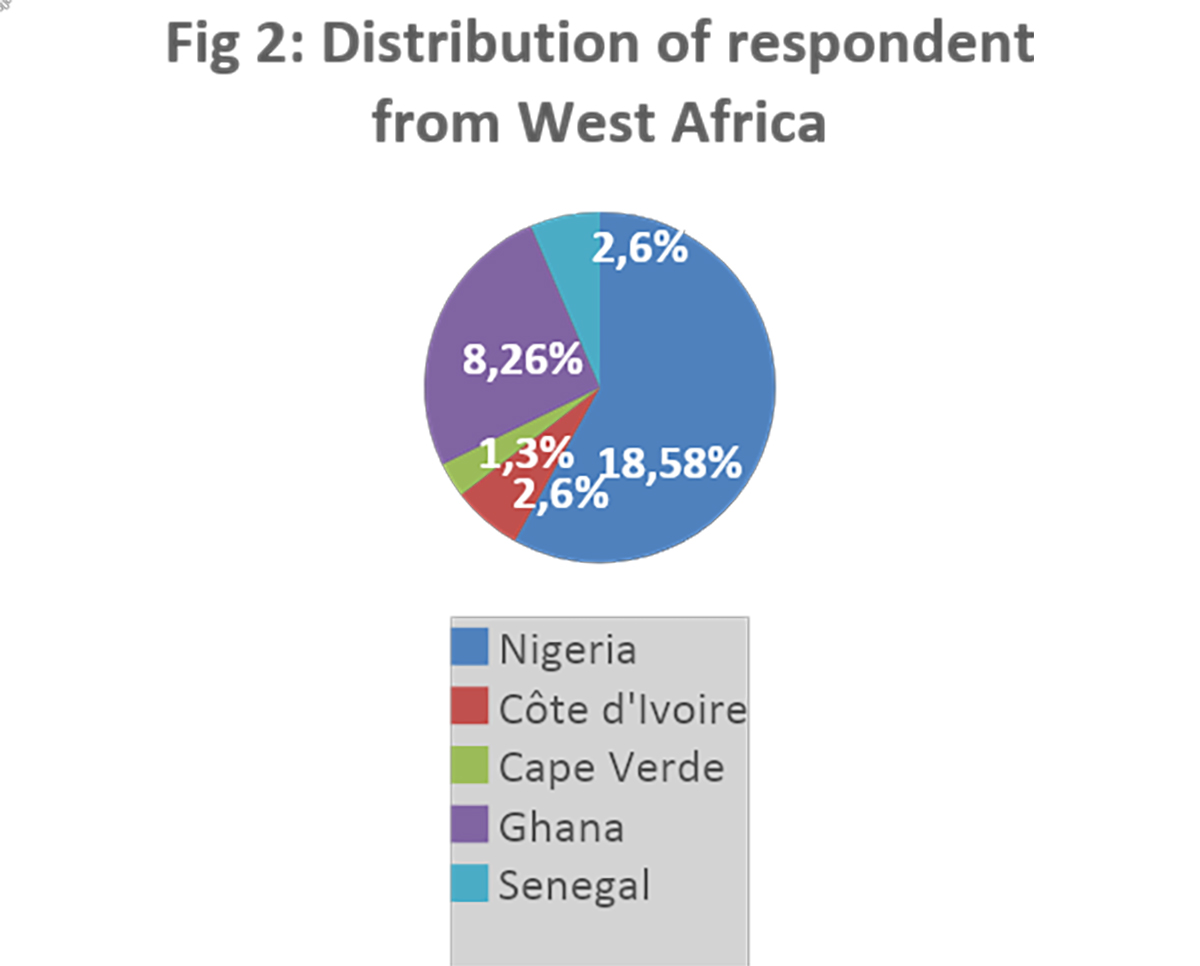
Industry contacts were obtained from industry associations in Africa and globally. The data was collected and analyzed in Microsoft Form and Excel. Sixty (60) responses were collected after the two-month survey period, representing a participation rate of approximately 24 percent.
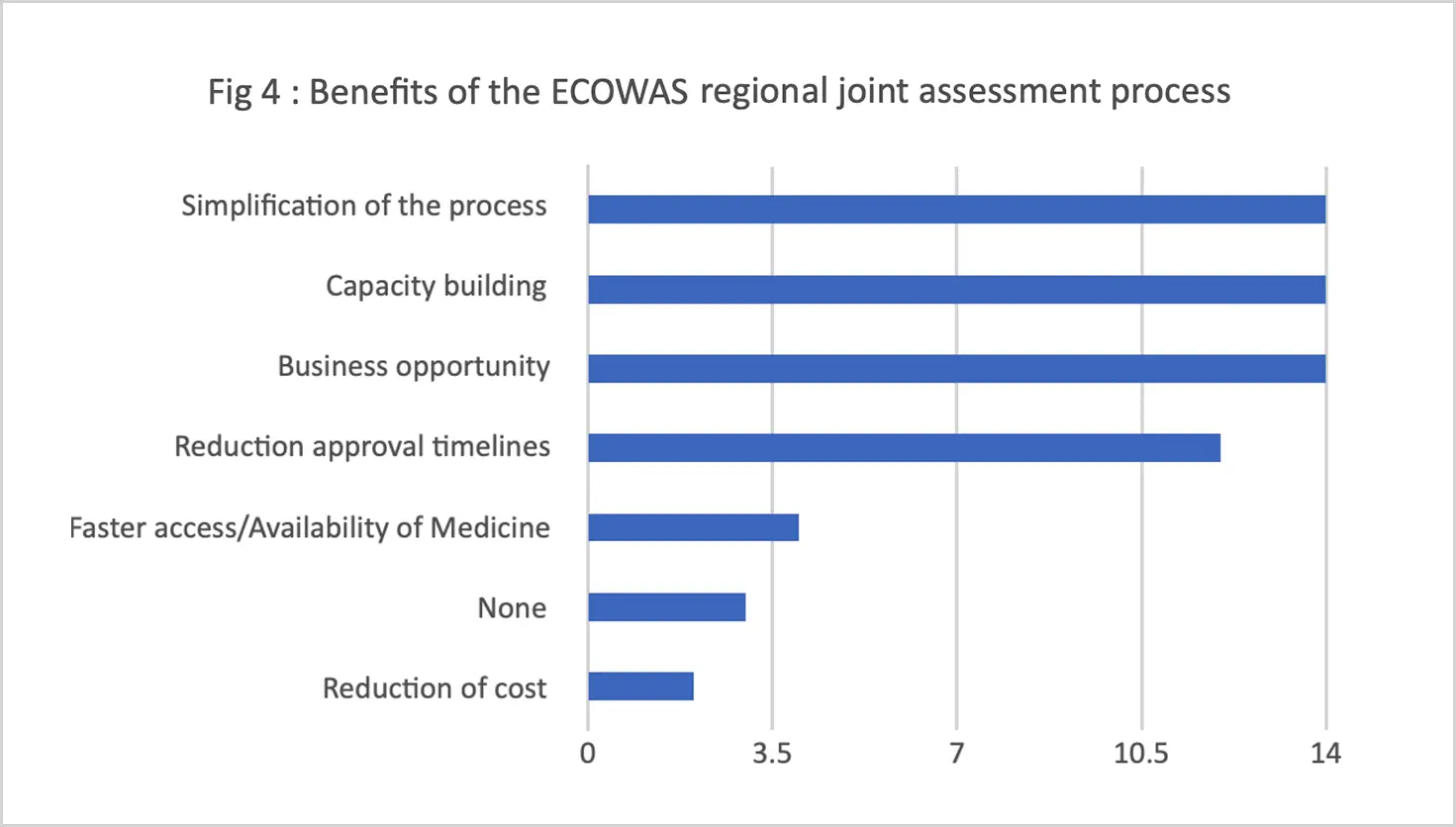
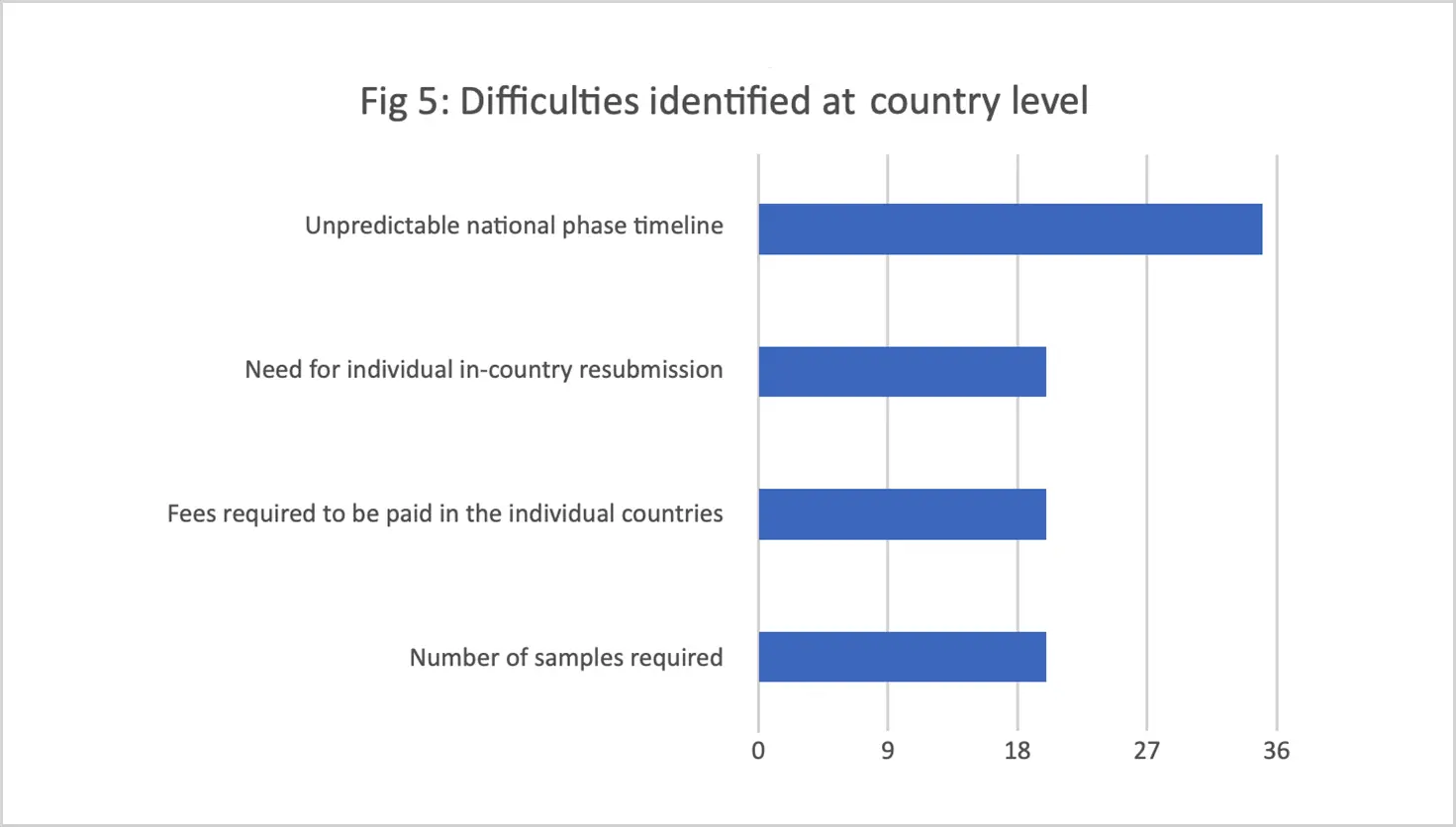
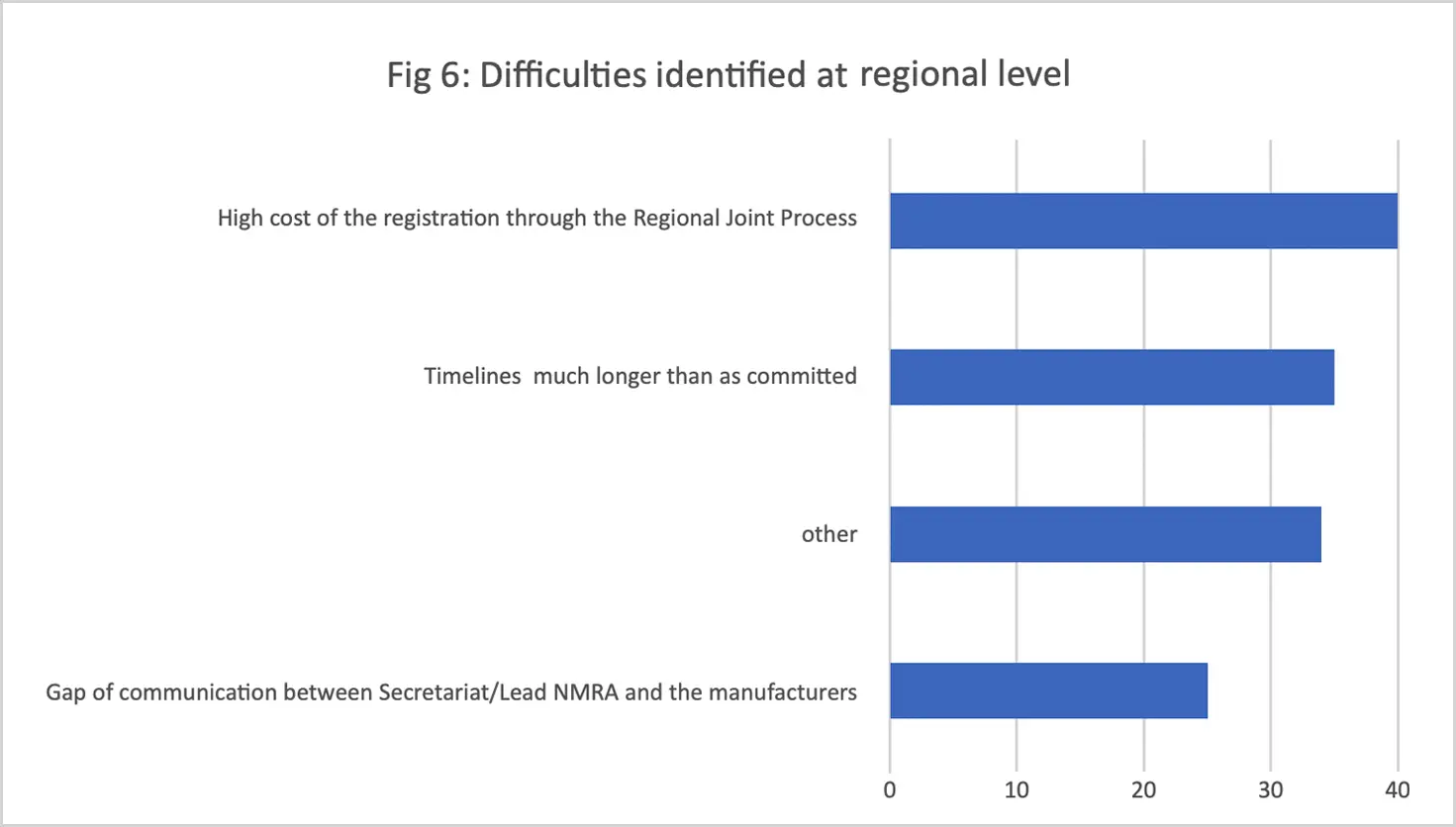
Challenges identified included lengthy timelines for both regional and national levels of the procedure, the high application fee to participate in the process, the varying (and in some cases unreasonably large) number of product samples requested by different national regulators, and inadequate communication between stakeholders. (The WA-MRH Steering Committee has since harmonized the number, timing, and disparities of samples required for testing by national regulators.)
In addition to optimizing the regional step to meet timeline commitments, WAHO is now actively following up with national regulators to ensure timely registration decisions after the company notifies the WAHO secretariat that the national filing has been completed; it is expected that timely national administrative actions will become institutionalized. In 2024, WAHO also plans to establish a platform that links regionally recommended products to pooled and national procurement through which these products will be recognized as quality products and preferred by regional and national procurers.
Conclusions
Applicants who use the WA-MRH procedure pay a single fee (between $8K and $12K) which covers both the assessment of the product and inspection of the manufacturing site. The WA-MRH Steering Committee has determined three different fees in this range, depending on the location of the applicant. This regional fee is unlikely to change, and WAHO believes that industry recognizes the value provided by this regional approach. While this is a modest fee for a service that provides market access to a region of 15 countries and close to 370 million people, we are now five years into this process and every pain point must be eliminated to enhance its value.
The WA-MRH Secretariat must explore more sources for disseminating and sharing information to make the benefits of this regional procedure better known to manufacturers and other stakeholders.

