Around the Globe
The Philosophy and Pathway for Implementation of Regulatory Science in Pharmaceutical Regulation—PMDA’s 10-Year Record and Prospects
Chief Executive Emeritus
Pharmaceuticals and Medical Devices Agency, Japan
n recent decades, progress in medical science and healthcare has been rapid, and people’s expectations of medical treatment have risen accordingly. However, some expectations have been blunted by unexpected adverse events associated with new drugs. For example, cases of HIV/AIDS and HCV/hepatitis in the early 1980s were associated with the use of coagulation factors derived from unheated plasma. Such events led to a cautious approach to the approval of new drugs. This in turn resulted in a long-standing issue of late approval of drugs and medical devices in Japan, compared with Western countries, and the terms drug-lag and device-lag were coined to describe the time lag between approvals of the same new product in Western countries and in Japan. In addition, public criticism of inadequate post-marketing safety measures for medicines led to calls for administrative reforms.
Developing Academia-Industry-Government Partnerships
Twelve years ago, PMDA adopted the following mission statement: We aim to pursue the development of medical science while carrying out our duty without delay, with greater transparency based on our absolute mission to protect public health and the lives of our citizens. Specifically, we aimed to speed up review and safety assurance measures in order to introduce new drugs and medical devices in a timely manner from a social welfare point of view, while at the same time enabling continuous and rapid monitoring of safety. To do this, we needed to break the conventional view that academia-industry-government should be independent of each other and instead promote academia-industry-government (AIG) collaboration. To avoid any public impression of potential impropriety, the principles of fairness, transparency, and ethics were considered essential.
Therefore, moving away from the idea of traditional authoritarianism, regulators actively introduced regulatory science indicators into regulatory operations. Figure 1 schematically illustrates this transition from simple evaluation science to more integrated regulatory science engineering (RS engineering). In short, RS engineering aims to reduce the disadvantages of reliance on any single aspect of science and technology output (“silo” mentality) and to promote more balanced, cooperative judgments.
As a part of this process, we trained more reviewers, with an emphasis on physicians, as well as pharmaceutical scientists and engineers, under the AIG partnership. The growth in the number of reviewers and their training was supported based on the principle of fee for service from industry.
In collaboration with the graduate schools of various universities, PMDA has established courses that specialize in regulatory sciences, including medicine, pharmacy, and medical engineering. In February 2016, this deepened into a comprehensive cooperation agreement—including institutions such as RIKEN, which encompasses a network of world-class research centers within Japan—as well as various highly specialized medical centers. Regulatory science received increasing attention in the medical community from the viewpoint of new product seed development, and this promoted the exchange of experts between academia and PMDA.
In April 2008, the number of PMDA staff was less than 450, but the total has now reached nearly 1,000; the number of medical doctors has increased from 25 to nearly 70; and PMDA now has access to experts from cutting-edge medical and research institutions on a regular basis.
PMDA also established the Science Board to bring together researchers who are directly involved in advanced studies leading to the development of innovative medicines. The board summarizes evaluation reports on advances in medical science and technology, in collaboration with PMDA’s reviewers, providing the foundation for horizon scanning of potential medical advances.
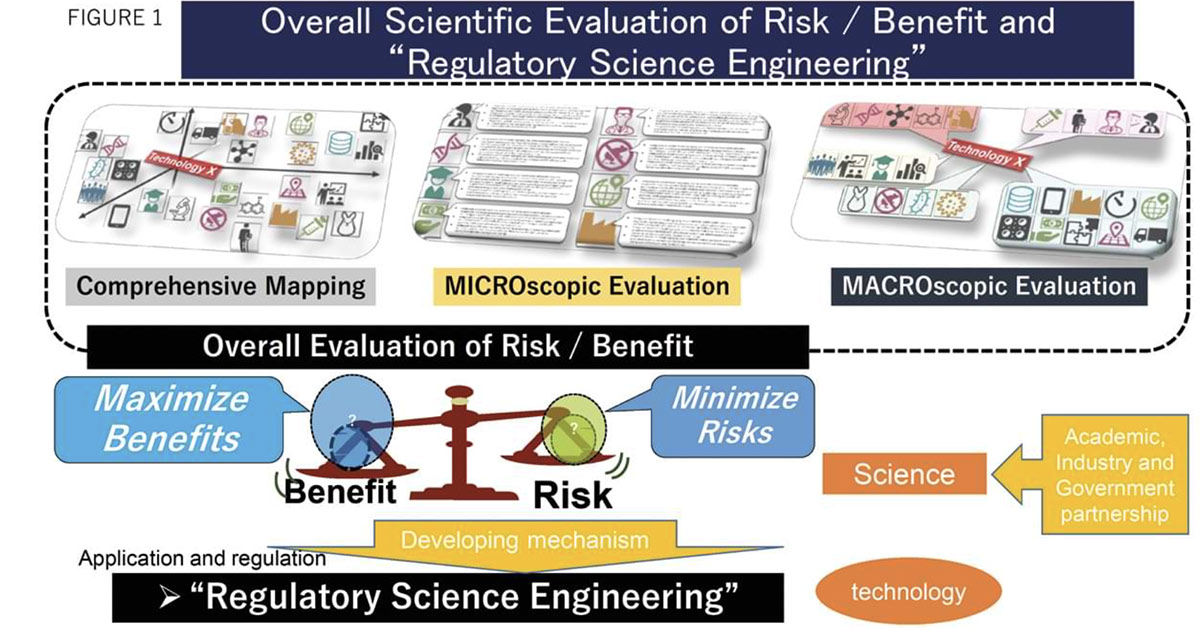
Outcomes of Regulatory Science
PMDA has improved its global standing among regulatory authorities by building up its scientific capabilities to accelerate new drug review and safety measures, demonstrated by the world’s fastest review speed for new drugs with new active ingredients for three consecutive years from 2014 to 2016.
After helping Japan overcome the issues of drug- and device-lag, PDMA introduced a reorganization of Japan’s clinical trial environment and other local measures, coupled with progress in global clinical development, such as multiregional clinical trials (MRCT) including Japanese subjects. PMDA has also played an active role in the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the International Coalition of Medicines Regulatory Authorities (ICMRA). Other international activities include establishing the Asia Pharmaceutical and Medical Device Training Center in April 2016 to train the staff of regulatory authorities in Asian countries.
Having improved reviewers’ knowledge and skills due to the introduction of the Science Board, PMDA developed a pharmaceutical strategy consultation program to provide special regulatory consultations for academia/small biotech business entities to promote AIG collaboration for clinical development of pharmaceuticals and medical devices. These special regulatory consultations have contributed to shortening the time for patient access to innovative medicines in Japan. Based on regulators’ experience of the AIG collaborative partnership, PMDA proposed and introduced the world’s first regulatory category for regenerative medical products (cell, tissue, and gene therapies) and a new system of conditional early approval for medicines. PMDA is the first regulatory authority to demonstrate the value and significance of regulatory science, distinct from conventional academic science (Figure 2).
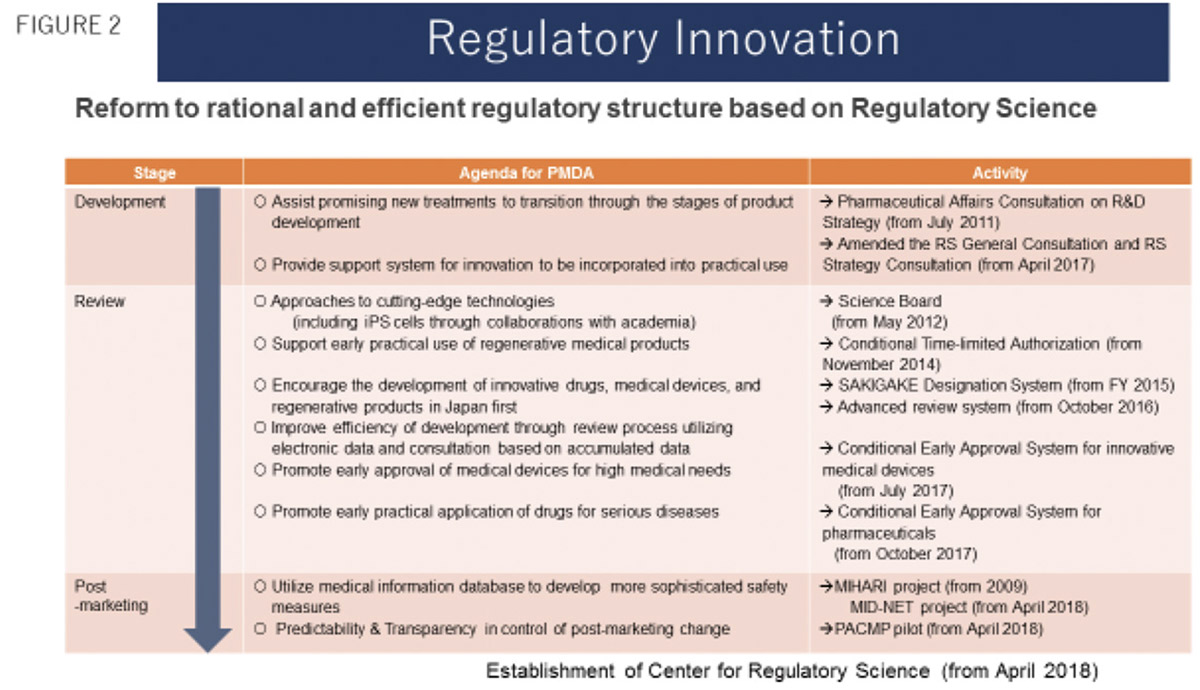
Prospects for PMDA’s Implementation of Regulatory Science in Pharmaceutical Regulation
I believe policy initiatives in medicine must be patient-centric, while making full use of scientific knowledge and methods. Early work in applications of science to policy-making or specific public policy planning has been reported in the US, and a new paradigm of socially distributed, application-oriented, transdisciplinary knowledge production, in contrast to the previous focus on academic science, has been proposed.
In other words, this new mode of science aims to improve human well-being by accounting for social and other factors as well as scientific findings. The original aim of regulatory science, as advocated in Japan by Mitsuru Uchiyama in 1987, was to study how to evaluate and predict the progress of science and technology by balancing the advantages and disadvantages in order to regulate matters most effectively for the public benefit. RS engineering was developed as a mechanism for implementing regulatory science decision-making along these lines, and we expect that this approach will maximize the effectiveness of medical care in the future.
I believe regulatory science can be regarded as a science that pursues relative values in a multidimensional and ethical way that will be broadly acceptable to human society. In other words, we would like to move away from the idea that science is a purely “intellectual” activity that searches for absolute facts or knowledge, and instead focus on the idea of a broader and deeper “intelligent” aspect of science that takes account of relative values including natural and social sciences and humanities, based on the social needs of humankind. Thus, we believe regulatory science should progress by incorporating both “intellectual” and “intelligent” aspects not only of the natural sciences but also of the social sciences and humanities.
In other words, we think we need a multidimensional approach that incorporates research on ethical, legal, and social implications (ELSI), which have received increasing attention in recent years, especially in the context of regulatory science. We anticipate that accounting for these social values will increase the efficiency and productivity of regulatory science, as well as its contribution to human wisdom. This approach may also help to stem the spread of pseudoscience, a substantial problem from a public health perspective (for example, the supposed cures for COVID-19). In this way, we believe a more intelligent regulatory science will be more effective in promoting the welfare of humanity in the future.
Conclusion
PMDA’s development work in regulatory science has aimed to fuse science and engineering into a new form of evaluation science (RS engineering) and to fully account for the need to establish common appropriate assessment methods for new drugs and devices that ensure rapid introduction when this is in the public interest, while also maximizing safety through ongoing monitoring. As a part of this work, it is important to harmonize international scientific norms and evaluation methods. Regulators must work closely with academia and clinical practitioners to establish common appropriate assessment methods. In particular, we wish to promote a multidimensional approach to regulatory science: an intelligent approach, incorporating research on ethical, legal, and social aspects. We believe such an approach will enhance the social implementation of new outcomes of science and technology for the benefit of humanity. The need for such measures has been clearly illustrated by recent work to develop, test, and approve COVID-19 vaccines.
DIA thanks Tatsuya Kondo, who passed away on September 26, 2021, for his profound contributions to regulatory science. We also wish to thank Daisaku Sato for coordinating the publication of this article.
Read our memorial tribute to Tatsuya Kondo.
The opinions expressed in this article are those of the author and do not necessarily reflect the official position of the PMDA.

