Around the Globe
rotecting and promoting public health in a time of increasing globalization with unprecedented advances in technology and medicines is the single greatest challenge facing medicines regulatory authorities today. COVID-19 effectively illustrated this point. Regulatory bodies and other stakeholders were forced to think creatively about adopting new technologies, sharing resources and expertise, and working more efficiently on a global scale. Many view regulatory cooperation among health authorities as imperative to navigating these rapid therapeutic advances in both emergency and nonemergency situations. Various forms of regulatory reliance and cooperation have developed in recent years. This article reports on Canadian industry experience with one such consortium, the Access Consortium (previously called ACSS).
Background
The consortium’s goal is to maximize international cooperation between member jurisdictions, reduce duplication, better align regulatory systems, and increase each agency’s capacity to ensure that patients have timely access to high-quality, safe, and effective therapeutic products. Heads of these agencies meet twice a year to continue building on their learnings and to define the strategic direction for their future work. Each agency also nominates a coordinator who serves as primary contact and ensures effective communication and meeting planning.
The New Active Substance Work-Sharing Initiative (NASWSI) within the Access Consortium was created in 2018 to focus on the review of submissions for innovative molecules that include extensive nonclinical, clinical, and technical manufacturing information. (In this paper, NASWSI and the Access Consortium pathway are used interchangeably to describe the work-sharing procedure.) This initiative was launched as a pilot in April 2018 with Canada and Australia sharing the review work and Singapore and Switzerland acting as observers. It is important to emphasize that work sharing was never intended to replace the independent regulatory decision-making of each authority. Expedited pathways such as priority review applications are not excluded from consideration, but all participating health authorities must accept the package as a “priority review status.” A call for applicants in Phase 1 of the initiative highlighted the potential for similar market authorization dates between regions and consolidated questions to applicants.
Review under the Access Consortium is initiated by sponsors by submitting an “Expression of Interest” (EOI) application form outlining any differences foreseen in the dossiers to all applicable health authorities. Participating regulatory authorities and the sponsor may conduct a joint meeting to lay out a consolidated roadmap for the review cycle, including dates for the initial list of joint questions and the proposed decision date. At this point, the principal review activities are divided, with Common Technical Document (CTD) modules 3, 4, and 5 assigned to different participating health authorities while country-specific CTD module 1 and the risk-management plan are independently reviewed by each authority. Despite the challenges posed by time zone differences, these initial steps can realize some efficiencies, as one pre-submission meeting can be planned for up to five countries simultaneously. Although the regulators are reviewing essentially identical dossiers (with minor differences elucidated in the EOI form), each participating country must submit a local package in electronic common technical document (eCTD) format, including:
- full toxicological, clinical, and quality information
- local adaptation of documents/requirements
- local proposed product labeling
- full local submission fees
Experience with the Access Consortium work sharing has grown since the inception of this initiative. On March 23, 2021, Swissmedic announced that the consortium had completed the review of its first application where the tasks were divided between the four health authorities and announced in late 2021 that two applications were under review with all five health agencies participating in the review. The consortium had approved 12 submissions through this work-sharing pathway as of October 2021, with an additional 10 submissions under active review and six EOIs under consideration.
Sponsor Experience with the Access Consortium
In June 2021, Innovative Medicines Canada undertook a survey of its members’ experiences to learn the sponsor’s perspective on both the challenges and benefits of participating in the Access Consortium. The survey was sent to 50 companies, of which 15 responded. These responses reflect experience with 14 submissions submitted through the NASWSI (including 100% [all eight] of the completed reviews in which Health Canada had participated by that time).
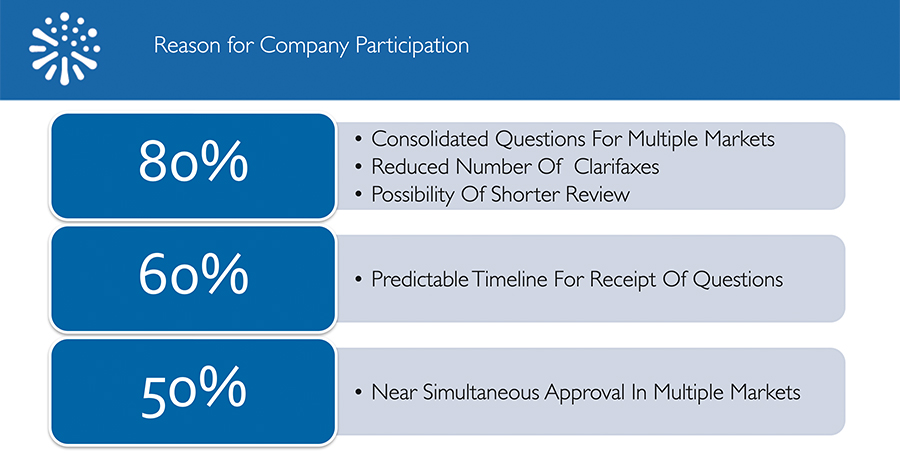
When asked what had motivated their participation in a NASWSI review, 80% of respondents selected the following reasons:
- Consolidated questions for multiple markets
- Reduced number of clarifaxes/clarimails
- Possibility of shorter review time
Approximately 60% of respondents cited predictable timelines for receiving questions as a reason for participation, and half (50%) cited the potential for near-simultaneous approval in multiple markets.
One company reported receiving five rounds of clinical questions, each with a timeline of only 15 days to respond. Other companies reported that the consolidated questions often included a list of country-specific questions comparable in length and complexity to the list of joint questions. These types of experiences likely contributed to our finding that while 70% of respondents felt that the process and milestones were clearly communicated to them up front (at the pre-filing planning meeting), only 25% reported adherence to these original processes and milestones.
When asked what barriers companies experienced or anticipated that made them reluctant to file a submission through the Access pathway, 75% reported that participating would pose a risk to a timely filing in Canada because other Access countries have a lower priority than Canada in their global filing sequence. Other relevant factors for filing decisions mentioned were:
- Lack of clear guidance, predictable process, or timelines
- No explicit incentive (e.g., guaranteed shorter review timeline or reduced fees)
- Anticipated extra effort/resources required for coordination between countries
- Lack of alignment on priority review among Access regulatory agencies
- Concerns regarding divergence among Access members related to technical requirements to support approval and risk/benefit evaluations
- Lack of clarity on how a parallel Health Canada/HTA review would be managed/operationalized within the context of Access, to ensure that reimbursement recommendations are accelerated for Canadians.
Since our survey was conducted (June 2021), the Access Consortium has issued NASWSI Operational Procedures which may address some aspects of the concern expressed in the first bullet above. The procedures themselves, though, outline the possibility of rolling questions, as opposed to the single consolidated list of questions. This lack of predictability on what industry views as one of the major benefits Access offers may further deter some companies from using this pathway.
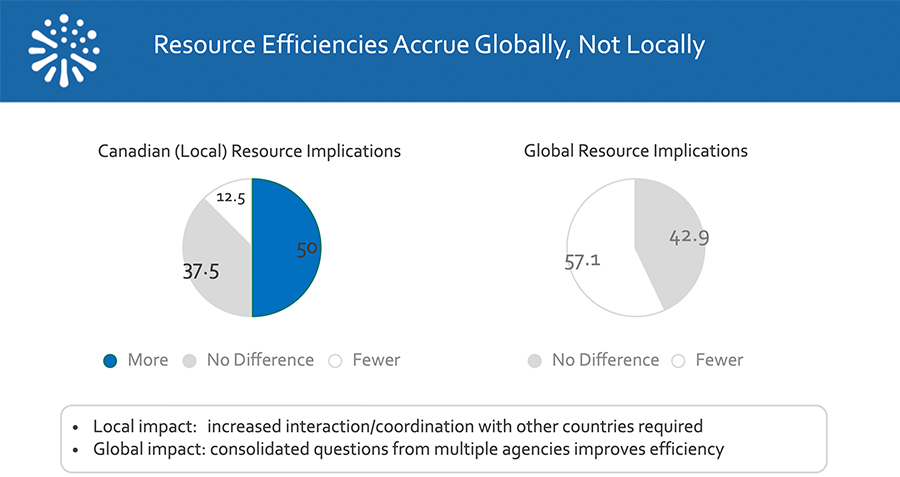
Survey respondents were also asked to indicate what impact a review through the Access pathway had on resource requirements, both within local Canadian organizations and for their global counterparts, as shown below.
Five of the eight submissions in our survey that had completed review reported receiving their decision ahead of the standard target date, while three received their decision on the target date. No company reported a delay in receiving their decision. Earlier decisions are not reflected in the official tracking system held by Health Canada and do not impact performance metrics. Overall, respondents indicated a positive experience with NASWSI, with 80% indicating that they would choose to follow the Access pathway again for another submission.
Recommendations
Predictability
Sponsors would also like to be assured of consistently shorter review times. While the results to date have been largely positive, it is hoped that as the collective experience grows, and health authorities are truly work-sharing to the most reasonable extent possible, the reduction in workload for each member health authority should translate into consistent and predictable reductions in review timelines, regardless of which countries are participating and which country takes the lead on any particular module.
Harmonization
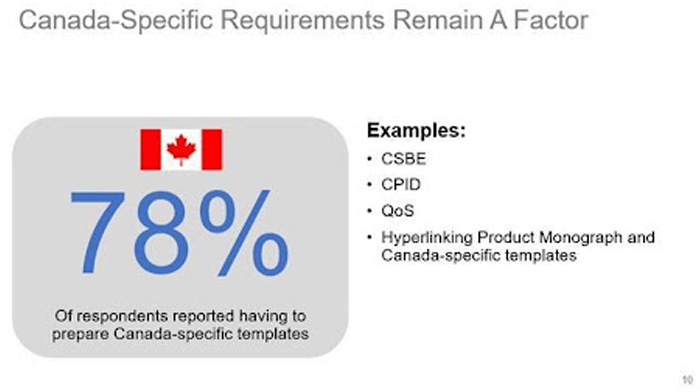
In addition, if the true goal of this process is to learn from each other and work toward convergence, there is still room for improvement: Canada-specific documents/templates (e.g., Module 1 CS-BE requirements, Module 3 regional documents), which can slow down the preparation and submission timelines especially for smaller companies (as shown in the recent CIRS report), are still required.
Indicators of NASWSI Success
Participating health authorities appear committed to the success of this pathway and outlined in their recently issued Strategic Plan the metrics they will use to measure success toward their stated goal to become regulators of choice:
- increase in applications to Access at the same time or soon after being filed with other major medicines regulators
- increase in number of products made available to patients through Access
- increase in diversity of products assessed through Access
- decrease in average time to market for products assessed under Access
- reduced effort and duplication for both regulators and industry
- increased collaboration on the alignment of products with healthcare system needs that are made available to patients through Access
- increased collaboration on global GxP inspections
- increase in number of ICH guidelines implemented through Access collaboration.
Broader Policy Consideration
While the potential reduced review timeline through the Access Consortium is positive and motivating, this alone is insufficient to ensure faster patient access to new therapies. The Canadian reimbursement system for new medicines remains complex and highly unpredictable. Much greater coordination and alignment is required across the review and reimbursement system to capture the full benefits from the Access Consortium. This would involve close collaboration and engagement with health technology assessment agencies (CADTH and INESSS), the pan-Canadian Pharmaceutical Alliance (which deals with price negotiation), and public drug plans. Faster health authority approval does not equate to faster patient access. Closer collaboration is needed to foster faster access for better patient outcomes.

