Around the Globe
CECMED Cuba
FIFARMA
he report of PANDRH’s Certificate of Pharmaceutical Product (CPP) Project represents the most far-reaching study carried out in the area, a step forward in closing the gaps, and a new opportunity to discuss the healthcare value of CPP in regulatory processes in the Americas.
The CPP concept has experienced challenges resulting at least in part from the evolution of science, regulations and international pharmaceutical markets. Because of this constant change, discrepancies based on different interpretations and uses of the CPP and triggered by the increasing complexity of the supply and manufacturing chains, new product types, regulations and legislation, and other factors, have caused different countries to apply the document differently.
To help contribute to discussion of CPP practices in the Americas, the Pan American Network for Drug Regulatory Harmonization (PANDRH Network) has approved the PANDRH CPP project, jointly coordinated by a regulator (the Centre for State Control of Medicines, Medical Equipment and Medical Devices [CECMED] in Cuba) and industry (the Latin American Federation of Pharmaceutical Industry [FIFARMA]). Complete information about this project was previously published by Global Forum. In 2020, the project reached an important milestone with the publication of a report that characterizes and identifies trends in CPP requirements and practices by regulators in the Americas.
The report was prepared in 2019-2020 with data collected in 2018 through a survey that contained 58 questions in ten sections. Data include requirements and practices related to the use of the CPP for submission of new drug applications, submission of renewal applications for post-approval changes or variations, the CPP format that is accepted and issued by different regulatory authorities, and the information evaluated in the CPP. It presents data for 27 countries, collected from regulators and industry representatives in the Americas (Argentina, Barbados, Belize, Bolivia, Brazil, Canada, Chile, Colombia, Costa Rica, Cuba, Ecuador, El Salvador, Guatemala, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Paraguay, Peru, Dominican Republic, Suriname, Trinidad and Tobago, Uruguay, the US and Venezuela).
Results identify the following trends among the 25 NRAs of the Americas that require the CPP for at least one type of submission:
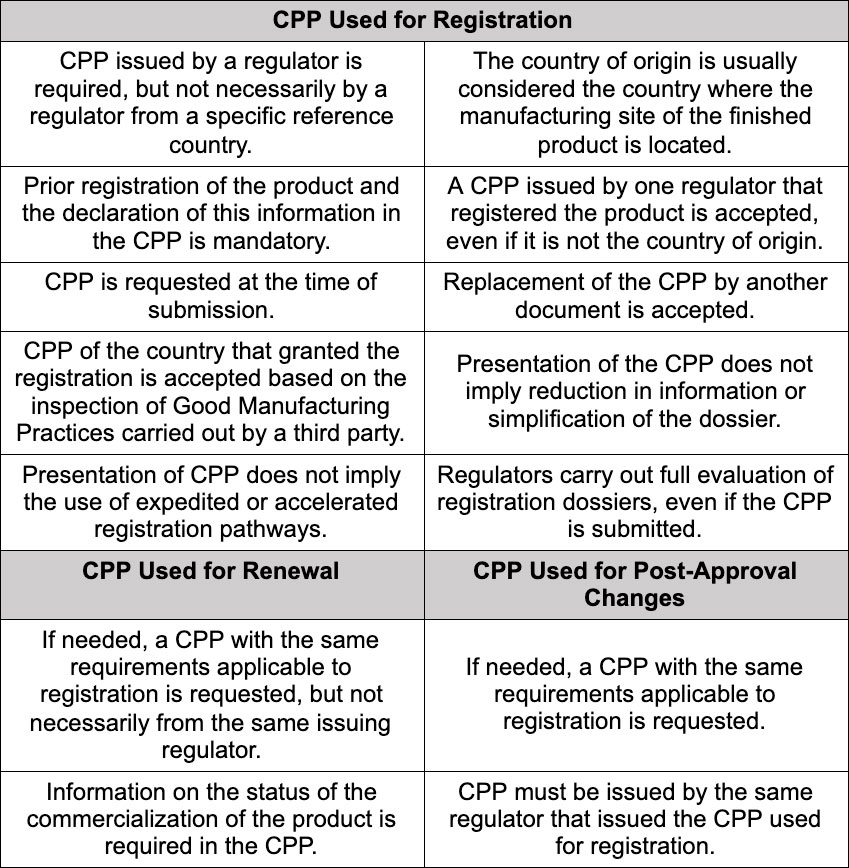
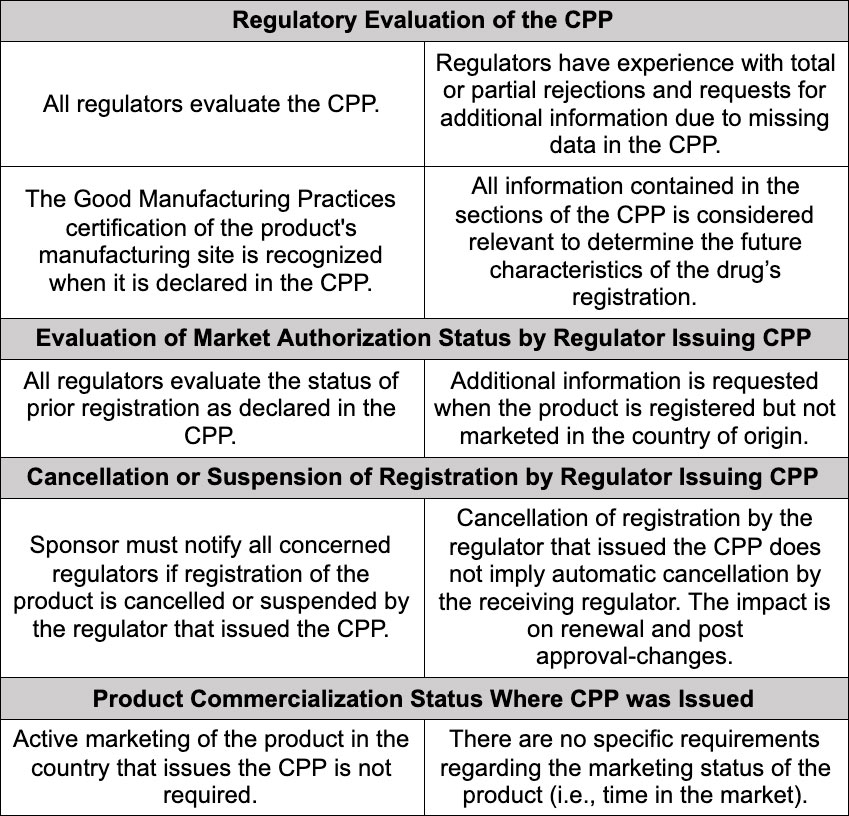
The report also indicated that there is a high degree of convergence between the characteristics of the CPP issued by regulators in the Americas and the WHO model; that issuance of an electronic CPP is still not general practice; and that the timeframe for issuing the document varies a lot but is usually between five and twenty business days. (Not all countries of the Americas issue CPP. Data refers to following countries: Argentina, Bolivia, Brazil, Canada, Chile, Colombia, Cuba, Ecuador, El Salvador, Guatemala, Honduras, Peru, Uruguay, Venezuela, and the US.)
Conclusions
Because the report illustrates the range of CPP practices and requirements in the region, and results from joint regulatory-industry effort, it is valuable for evaluating and refining regulatory systems to better meet current demands. Its publication coincides with WHO approval of its updated Scheme, a particularly important and timely action for the analysis and follow-up of the data presented in the report.
FIFARMA Report Released with Evaluation of the CPP Requirements in the Americas Region
Executive Report: Evaluation of the requirements of the Certificate of Pharmaceutical Product (CPP)
Complete Report: Evaluation of the requirements of the Certificate of Pharmaceutical Product (CPP)

