What’s the (End)Point in Digital Health Technology?
he use of technology in clinical trials has increased dramatically. Although technology is now used at all levels of the process, from molecule discovery, trial logistics, and participant selection, clinical trial endpoint measurement is also gaining traction, with substantial growth in recent years. In fact, 378 unique digital endpoints are currently being used by 104 sponsors across a wide range of therapeutic areas. But some challenges still stand in the way of fully describing digital endpoints and measures in drug labeling.
Barriers of Our Own Design?
The evidentiary hurdles and burden of evidence facing digital endpoint development are high, so it is important to have a strategic and structured approach to forming a patient-centered label claim from DHT measurement.
The Library of Digital Endpoints maintained by the DiMe Society shows a large range of DHT parameters currently being used in clinical trial research at various endpoint positioning levels (primary, secondary, and exploratory). Unfortunately, standardization is lacking not only in the consistency of DHT parameters selected for endpoint assessment but even in the terminology used for describing the intended effects. Without collaboration, explaining treatment effects to regulators, payers, patients, and clinicians becomes an arduous, if not impossible, task.
Barriers to the Future of Health
Initially, this lack of labeling seems somewhat incongruent with the utility of these tools to assess meaningful aspects of health: DHTs can measure more aspects of the disease and measure these aspects more often. They can perform as well as (or even outperform) traditional clinical assessments such as the six-minute walk test, the gait test, or the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). Using DHTs can lead to more specific and contextually relevant measurement, and measure aspects unavailable to researchers in the clinic, therefore increasing potential detection of a treatment effect which may be missed by more traditional means. Despite this, there are many barriers which could explain their perceived limited adoption by regulators, barriers which do not exist to the same extent for more traditional Clinical Outcome Assessment (COA) measures that assess the patient’s ability to feel, function, and survive: Barriers such as the timeline needed for creation of a DHT-based endpoint, which may not map to the clinical trial timelines, but also the developing frameworks we operate under when generating the supportive evidence. There is a need for agreement on these timelines and frameworks among many industry stakeholders, including internal teams (e.g., patient-centered outcomes research, digital biomarkers, clinical, regulatory, and DHT operations) and external bodies such as regulators and payers, healthcare professionals, patients, patient families, and patient advocacy.
Working Together to Break Barriers and Build Bridges
Pre-competitive collaboration between patient-centered measurement scientists, digital technology developers, academic institutions, regulators, payers, patients, clinicians, and pharmaceutical companies is sorely needed to identify standardized digital endpoints that all stakeholders can agree on. In a pre-competitive environment, stakeholders can work together to address foundational issues before moving into the competitive space. This allows the views of all stakeholders, particularly in the regulatory and payer space, to be brought onboard early in the process, which in turn ensures that the research is built on a bedrock of standardized terminology and scientific endeavor when it moves into the competitive space.
Framework for Pre-Competitive Collaboration
Pre-competitive frameworks are now a mainstay of the regulatory space. C-Path has led the way in hosting such collaborations, and the Digital Medicine Society leads these efforts including colleagues from the digital health technology development space. A typical pre-competitive collaboration will start with the identification of an issue that needs a solution while prioritizing the aspects that can be addressed as part of the collaboration. Next, this idea is refined and a research question is developed. This could be disease-specific (such as measurement in Alzheimer’s disease) or omni-therapeutic (such as measurement of sleep across many indications). Teams interested in the selected issue are convened to work in an environment of shared responsibility, and the shared scope is defined. From here, pre-competitive collaboration creates the bedrock of evidence needed to progress the field in a unified direction. Created resources are made publicly available, and the team drives adoption of the new resources to help standardize the field (Figure 1).
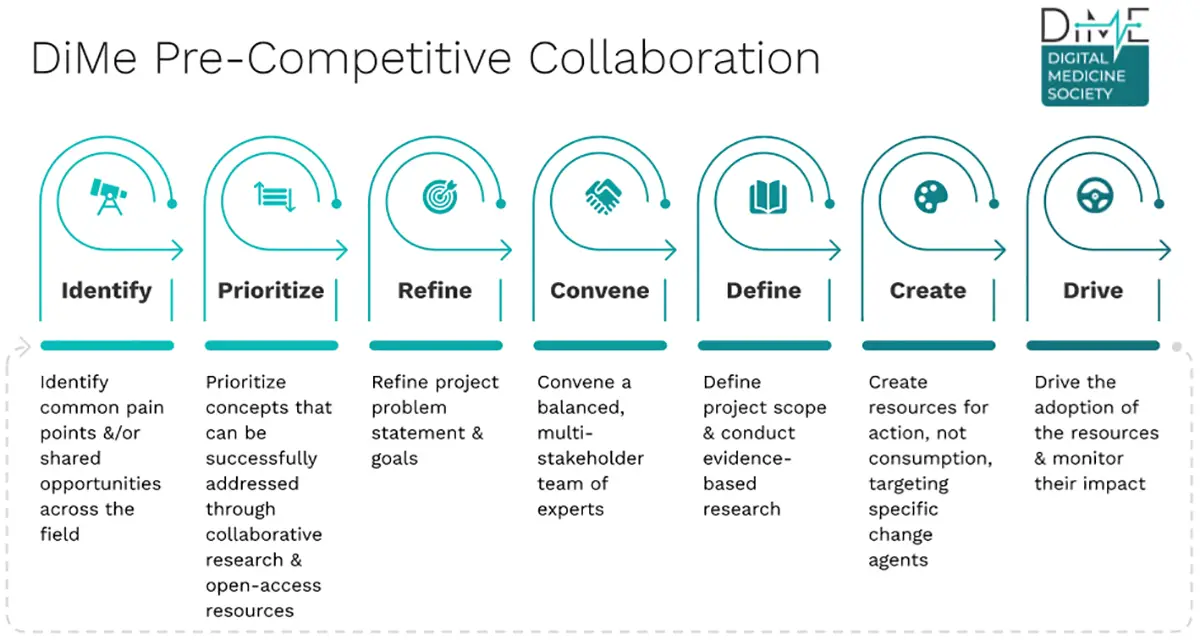
Creating for Action, not Consumption
When creating resources for action in line with the need for patient-centered digital endpoints, a pivotal task for the new collaboration is to understand the aspects of health and disease that matter to the patients they are intending to treat. This can be done in several ways, but typically will involve some qualitative or mixed-methods research supported by existing literature. This should be conducted in line with the well-established and accepted framework “digital measures that matter to patients.” This framework is important because it goes a step beyond traditional interview study methodology employed in the generation of patient-centered endpoints which looks to describe patient symptoms, and instead focuses on aspects of a patient’s disease that (a) they do not want to become worse; (b) they want to improve or; (c) they want to prevent.
Gleaning evidence in these three areas is an important departure from traditional qualitative work, as it allows for rich information about the patient’s expected treatment goals. This will both inform clinical trial endpoint design and offer rich evidence for the endpoints’ patient-centricity.
Once the aspects of health that are important to patients have been defined, the team can work together to:
- Generate conceptual models and the ontology or terminology to precisely define the aspects of health that matter to patients.
- Discover available digital parameters which may measure the discovered aspects of health. These parameters may already exist and have supportive evidence, or may be specified based on needs arising from the gathered evidence.
- Work with all stakeholders to link the patient-relevant aspects of health to the most suitable digital parameters while identifying any research gaps needed for further action.
- Make all evidence and resources freely available to encourage standardization.
Precedent: A Framework that Works
An excellent example of this work in action was conducted in dermatitis, specifically focused on nocturnal scratch. Following the above steps, a multistakeholder group generated an open resource to standardize and guide the field forward. This included a conceptual model, a reusable data set from a mixed-methods interview study, resources for clinical trial deployment, a set of foundational terminologies and ontologies, and resources for guiding payer acceptance and feedback from interactions with the FDA through a critical path innovation meeting. These collaborations clearly offer a rich source of knowledge for those involved and for the field moving forward.
There is still a large amount of diversity in the types of digital endpoints generated, the data collected, and the relevance of the evidence to patients. Working together, we will find firm and common scientific ground to help orient all stakeholders and ultimately lead to faster and more relevant clinical trial outcomes for clinicians and patients.

