Part 1: Reversing the Impact of Omitting More than One Billion People from Global Research
Innomas Clinical Research
Leslie Sam and Associates
Patient Safety Advocate, Kenya
Uganda National Drug Authority
International Society of Pharmacovigilance Africa Chapter
n 2010, the Deloitte Life Sciences and Healthcare Industry Group collaborated with the Economist Intelligence Unit on a white paper addressing the future of the life sciences industries after the 2008 and 2009 global recession. With feedback from senior executives, C-level executives, and board members, this paper highlighted the growing importance of emerging markets. China, India, Brazil, Russia, Mexico, and Turkey were identified as these emerging markets, but not a single African country.
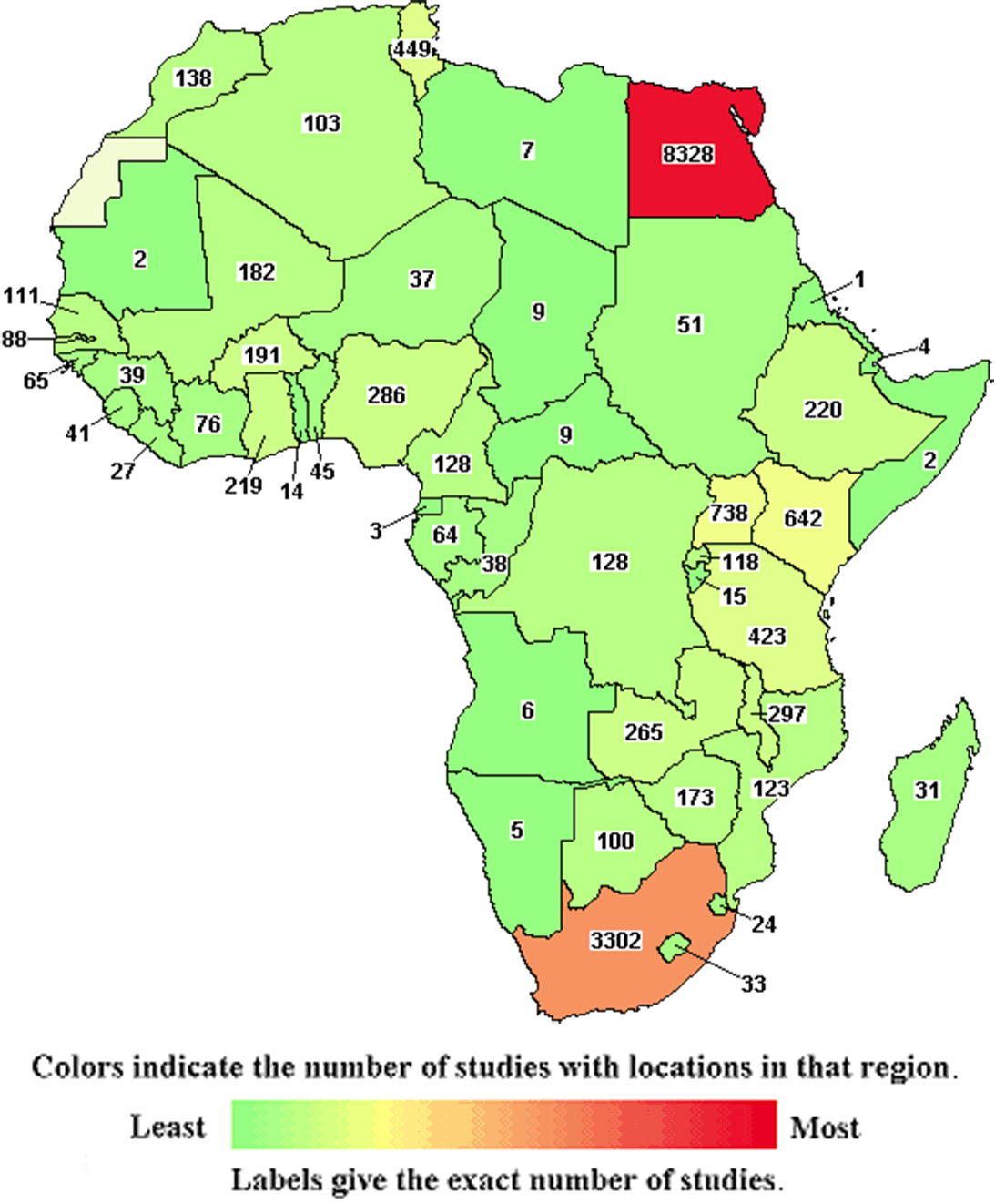
Africa is a Region NOT a Country
Fifty-five (55) countries make up the continent that is home to ~1.4 billion people, projected to be more than 2 billion by 2050 (the African Union recognizes Spanish Sahara as the fifty-fifth). The five most populous countries are Nigeria (~200 million), Ethiopia (~118 million), Egypt (~104 million), Congo (~92 million), and Tanzania (~61 million).
The Myths
The lack of understanding about Africa and its 55 countries, “in part due to lingering neocolonial attitudes in the Global North,” contribute to uneducated myths and assumptions about the continent. Failure of commitment to learn about the African continent commonly results in the practice of lumping all African countries into one monolithic unit that ignores the environmental and cultural differences not only between countries but also between states and regions within each country. The vastness of Africa makes for many diverse environments, from the most rural settings to cities that have a developed infrastructure on par with that of the West.
Reflections on challenges in funding and infrastructure, scientific and regulatory capacity and expertise, and in ensuring the safety of patients and the integrity of clinical trial data, continue below and in Parts 2 and 3 of this article series.
Bridging the Gap
Wangui Mathenge (International Society of Pharmacovigilance Africa Chapter) notes that drug development in Kenya and across Africa is unfortunately and significantly low despite the continent’s diverse population and practices, and offers several explanations:
- Lack of proper funding vital for strengthening healthcare systems in Kenya and many other African countries.
- Lack of expertise and/or robust regulatory framework that supports and protects drug development.
- Lack of credible Kenyan-specific or African-led scientific and/or medical data that would encourage sponsors and/or drug manufacturers to motivate drug development in the region.
“African patient data in global clinical trials has consistently been missing to date,” Mathenge explains. “As a result, some of these drugs that eventually succeed and enter the registration and post-marketing stage seriously lack the appropriate risk management plans and risk minimization measures for their products to the African population, all because Africa was missed during the drug development and research stage. Consequently, drug researchers and manufacturers miss recording other beneficial effects of their products experienced in the African population during the drug development and research phase.”
When considering Africa within the global clinical trial landscape, each country and region within each country must be assessed. Generalizations and pre-conceived ideas of the continent and its countries must be replaced with facts. The H3D Africa genomics project may offer helpful facts and insights.
The life sciences industry has taken the time to understand the environmental and cultural uniqueness of other regions to expand drug development and access; Japan, China, India, ASEAN, Latin America, and countries previously identified as the “emerging markets” provide prime examples of understanding and respecting cultural differences. This same effort and respect must be afforded to countries across Africa in assessing each country’s unique characteristics. The use of evaluation methods such as A.T. Kearny’s Overall Country Attractiveness Index for clinical trials (a method proposed by the US NIH in Current globalization of drug interventional clinical trials: characteristics and associated factors, 2011-2013) could provide better understanding of patient availability, cost efficiency, relevant expertise, national infrastructure, regulatory conditions, and other key topics.
As the prevalence of noncommunicable diseases such as diabetes, chronic respiratory disease, chronic kidney disease, cardiovascular disease, cancers, and mental and substance use disorders increases across Africa, the opportunities and need to expand research to include more than the communicable diseases (e.g., malaria, HIV, TB, Ebola, etc.) for which research on the continent is typically known are huge.
In her keynote address to the Global Congress on Oncology Clinical Trials in November 2018, Mojisola Christianah Adeyeye, the director general of Nigeria’s National Agency for Food and Drug Administration and Control (NAFDAC), noted: “The threat of cancer in our society today cannot be over emphasized as it has become a chronic disease claiming millions of lives every year. In Nigeria, the incidence, prevalence, and mortality of cancer has been on a substantial increase in recent times and has posed a tremendous burden on patients, families, and the society.”
Numerous African countries have laid foundations for clinical research. Institutions to govern and oversee research have been established to focus on improvements to decrease risks to the safety of their patients and integrity of research data, improving the quality of medicines and devices subsequently produced.
Regulatory authorities such as the Ghana Food and Drug Authority (FDA), the Pharmacy and Poisons Board (PPB) in Kenya, the National Agency for Food and Drug Administration and Control (NAFDAC) in Nigeria, the National Drug Authority (NDA) of Uganda, and the Botswana Medicines Regulatory Authority (BoMRA) (to name only a few); science-based registration requirements; bodies such as the African chapter of the International Society of Pharmacovigilance (IsOP); and the formation of unified organizations such as the African Medicines Agency provide examples of systems/frameworks established across the continent to strengthen mechanisms that ensure patient safety, rights, and well-being, along with data integrity. For example, as Ghanian Minister of Health Hon. Kwaku Agyeman-Manu explained in December 2022: “The experience we have gained from implementing the African Medicines Regulatory Harmonization (AMRH) initiative should not go to waste; it must serve as a catalyst to enhance the activities of the African Medicines Agency, which will soon become operational.”
These organizations are also committed to training staff to meet global standards. The Ghana FDA, in collaboration with the School of Public Health of the University of Ghana and AUDA-NEPAD, has engaged with regulatory officers from Liberia, Nigeria, Sierra-Leone, Zambia, Uganda, the Gambia, and Tanzania for advanced clinical trial training.
“Regulatory capacity is growing in all aspects of clinical trials, good manufacturing practice, post-market surveillance, and pharmacovigilance. There are regulations and guidelines in place,” explains Helen Ndagije of the Uganda National Drug Authority. “In Uganda, like many other African countries, the authority is simplifying the processes for drug development at all levels. The experience for more sophisticated trials has grown. Uganda National Drug Authority receives between 15 to 20 new clinical trial applications in a month, and the timelines for processing these have been drastically reduced, from about 65 working days in February 2018 to an average of 22 working days in July 2022 (possibly due to fast-tracking during COVID-19). The clinical trials unit handles about 90 percent of the applications within service delivery timelines. For conventional drugs, there are accelerated pathways to product registration. The authority has an interactive and informative website. The authority has an active Twitter account and routinely holds radio and TV talk shows.”
For decades, African life sciences professionals have been dedicated to advancing research to afford access to healthcare options to local patient populations that have historically been deprived of the benefits that research may offer.

