Around the Globe
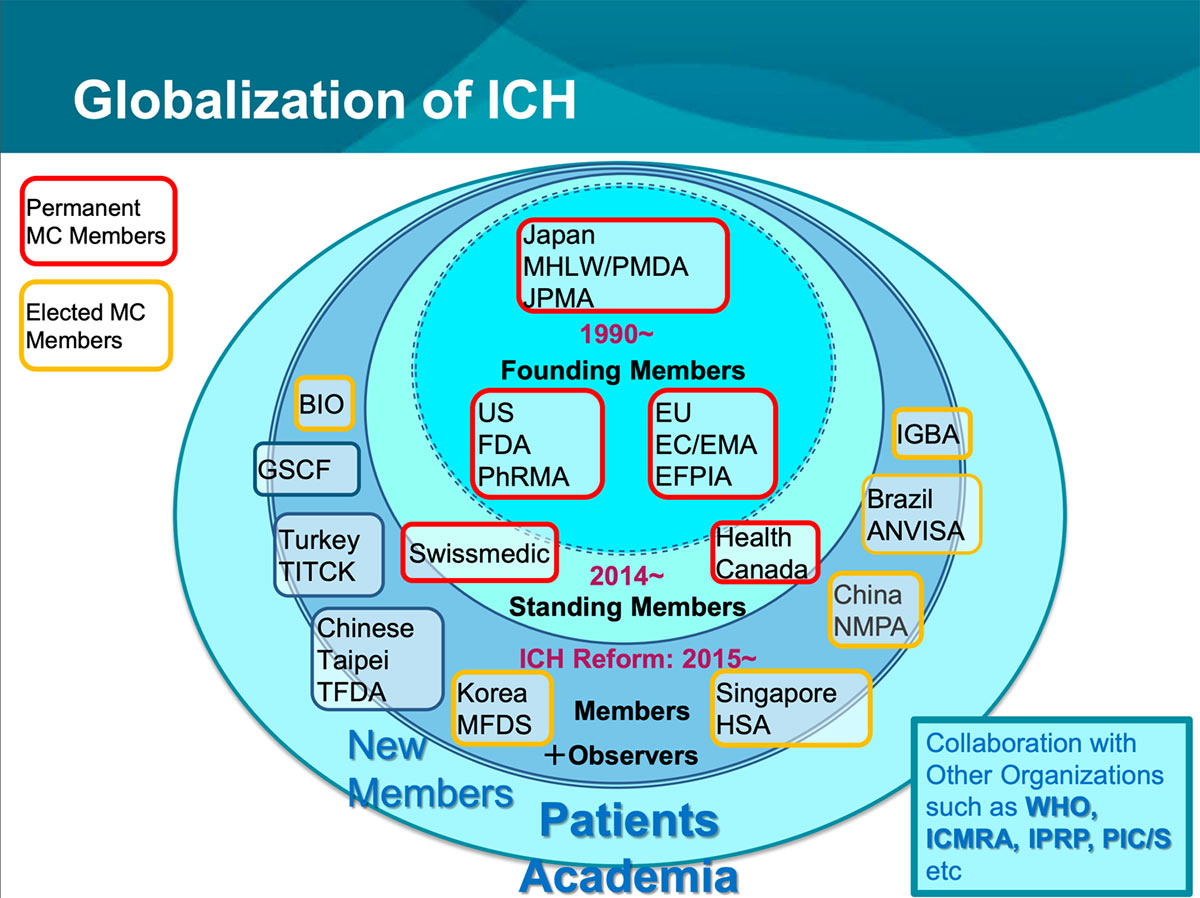
he International Conference for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) was established in 1990 to achieve greater harmonization worldwide to ensure that safe, effective, and high quality medicines are developed and registered in the most resource-efficient manner. In 2015, the organization and operations were renewed (this was called “ICH reform”) as a legal entity under Swiss law, and its name was changed to the International “Council” for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).
This renewal increased participants and made ICH a truly globalized organization (Figure 1). ICH activities for strategic development of technical guidelines, secure implementation of guidelines, and training for disseminating guidelines, are ongoing.
To reaffirm the achievements of ICH as we celebrate its thirtieth anniversary, and to reflect upon the future prospects for ICH mainly focused on the role of Japan, the 17th DIA Japan Annual Meeting 2020 focused numerous discussions on ICH-related topics.
ICH 30th Anniversary: Summary and Future Prospects with the Role of Japan
ICH has made tremendous contributions to efficient and simultaneous global drug development, standardized drug evaluation and review by regulators, and minimizing drug lag by facilitating earlier access to high quality innovative medicines. ICH has constructed systematic regulation among the world’s regulators and spread awareness that technical requirements and guidelines should be developed based on regulatory science without any politics. ICH leadership in these areas have provided clinical and regulatory experts with opportunities to work hard and encourage each other, further building this systematic network of experts.
ICH engagement with patients (among other stakeholders) has introduced a major movement leading to infrastructures that capture and utilize patient voice from drug discovery and development through post-approval measure and a related patient-focused drug development Reflection Paper on the ICH website.
ICH will continue to face challenges, such as accommodating new and innovative technologies and products as quickly as possible, in delivering superior treatment solutions to patients around the world.
Summary and Future Prospects in Area Q (Quality) and Role of Japan
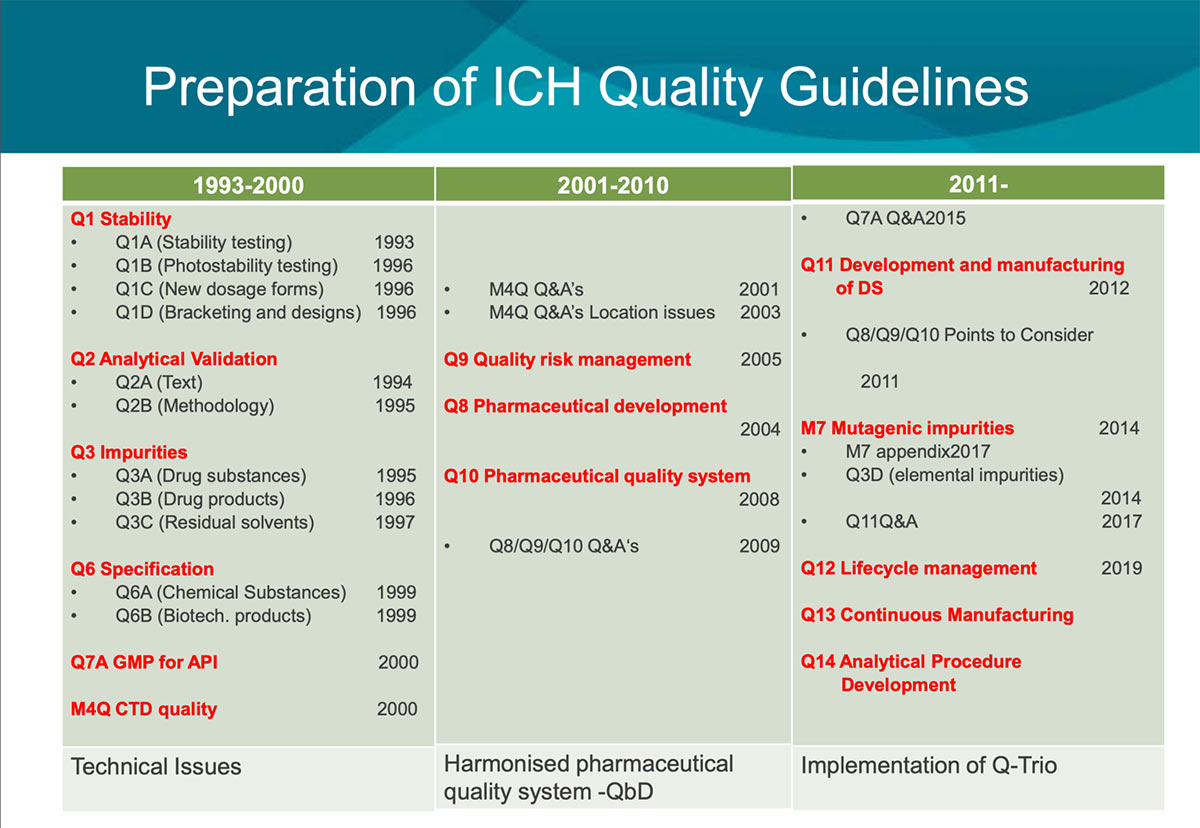
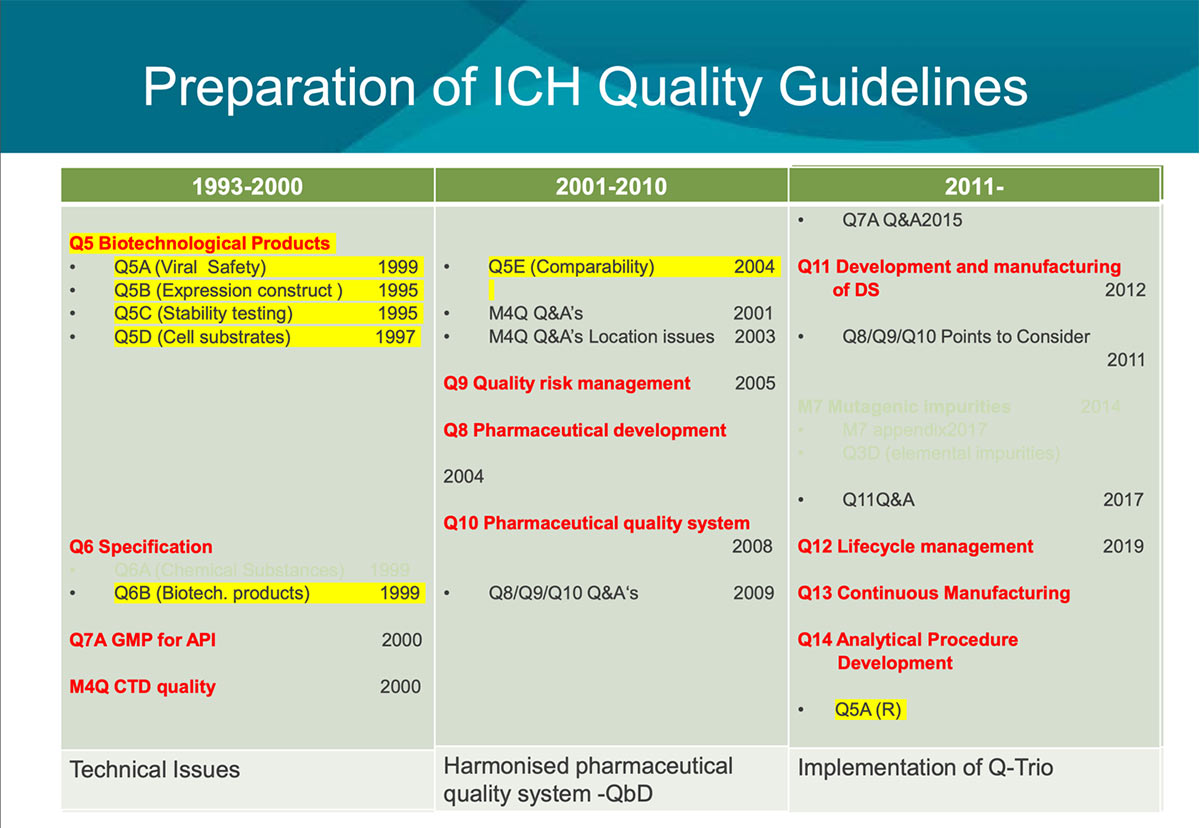
For the first ten years of ICH, basic technical guidelines such as the Stability guideline were developed in the Quality area. In the next decade, guidelines to promote the Quality-by-design concepts represented in the Q-trio guidelines (Q8, Pharmaceutical Development; Q9, Quality Risk Management; and Q10, Pharmaceutical Quality System) were developed. Technical guidelines for biologics have since been developed.
The most recently published guideline Q12, Considerations for Pharmaceutical Product Lifecycle Management, has made a great impact on Japanese regulation, especially changes in the Marketing Authorization application. PMDA has adopted a pilot program called PACMP (Post-approved Change Management Protocol) consultation to properly implement this guideline. The Q12 working group is developing training materials, including case studies and pre-recorded video clips, to help spread further understanding of this guideline. All stakeholders agree that respecting diversity and training are essential for harmonization. (See below for the history of Q series guidelines [Figure 2 and Figure 3]).
Summary and Future Prospects in Area S (Safety) and Role of Japan
ICH guidelines S1 (Rodent Carcinogenicity Testing), S5 (Reproductive Toxicology), S6 (Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals), and S11 (Nonclinical Safety Testing in Support of Development of Paediatric Pharmaceuticals), have led to remarkable progress and advanced discussions in these areas, including where and how their scope applies to oligonucleotide therapeutics and vaccines.
Japan’s important contributions to ICH safety guidelines include proposing the topic and conducting the data survey on histopath and immune function for S8 (Immunotoxicity Studies for Human Pharmaceuticals), developing the ROS assay system for S10 (Photosafety Evaluation of Pharmaceuticals), and proposing the topic for S12 (Nonclinical Biodistribution Considerations for Gene Therapy Products). Japan looks forward to proposing new topics that correspond to emerging modalities and to become a Rapporteur to lead this area.
Summary and Future Prospects in Area E (Efficacy) and Role of Japan
Since the E5(R1) guideline, Ethnic Factors in the Acceptability of Foreign Clinical Data, was published in 1998, Japan has the most advanced experience in planning and reviewing bridging studies. As a result, many sponsors companies have been able to reduce their clinical trials in Japan and Japan’s regulatory authority has been able to utilize much foreign clinical data.
Implementation of E5 was also a good experience because it has allowed Japan to participate in many simultaneous global clinical development programs. Facilitated by the E6 Good Clinical Practice and E8 General Considerations for Clinical Trials guidelines, terms and rules in clinical trials have been made so common and universal that they form the basis for accepting data from foreign multiregional clinical trials (MRCTs). Considering the importance and increasing use of MRCT, MHLW/PMDA proposed MRCTs as the E17 topic and led discussion as a Rapporteur. Through these and similar experiences, Japan has become comparable to the US and Europe in pharmaceutical regulation. Japan will continue to engage with other countries in improving ICH guidelines according to technological progress.
Summary and Future Prospects in Area M (Multidisciplinary) and Role of Japan
Experts from Japan and many other countries have devoted their work in this area to developing electronic standards suitable for pharmaceutical regulations. These standards contribute to a “common language” across ICH regions and make communication between regulators and industries, through eCTD and other digital regulatory submissions, more efficient.
Although ICH initially developed the M2 standard directly, M2 currently adopts the Standards Development Organization (SDO) process, which utilizes standards developed by an external organization. These standards were not originally developed for ICH, and ICH experts are not necessarily familiar with them. M2 continues to discuss improving the standards development process and how to determine which electronic technologies to accept from the vast, emerging technologies landscape and introduce into ICH
ICH has accomplished all the above and more over the past thirty years. These accomplishments have built global confidence in the pivotal role that ICH plays in the regulatory arena and other global systems.