Around the Globe
Roche Diagnostics
Stryker
APACMed
Diagnostics Development Hub
Diagnostics Development Hub
Roche Diagnostics
he Asia-Pacific Medical Technology Association (APACMed) is the trade association for the MedTech industry in Asia-Pacific. Following the 2020 launch of its Digital Health Committee, APACMed is seeking greater harmonization on topics such as regulation, interoperability, and cybersecurity in digital health.
This paper reviews regulatory measures for digital health (DH) solutions in Singapore, Australia, Japan, and the US, and proposes a best practices framework for regulators to use as they implement fit-for-purpose regulation for DH solutions.
Overview of Digital Health Regulation in Asia-Pacific: Best Practices and Gaps
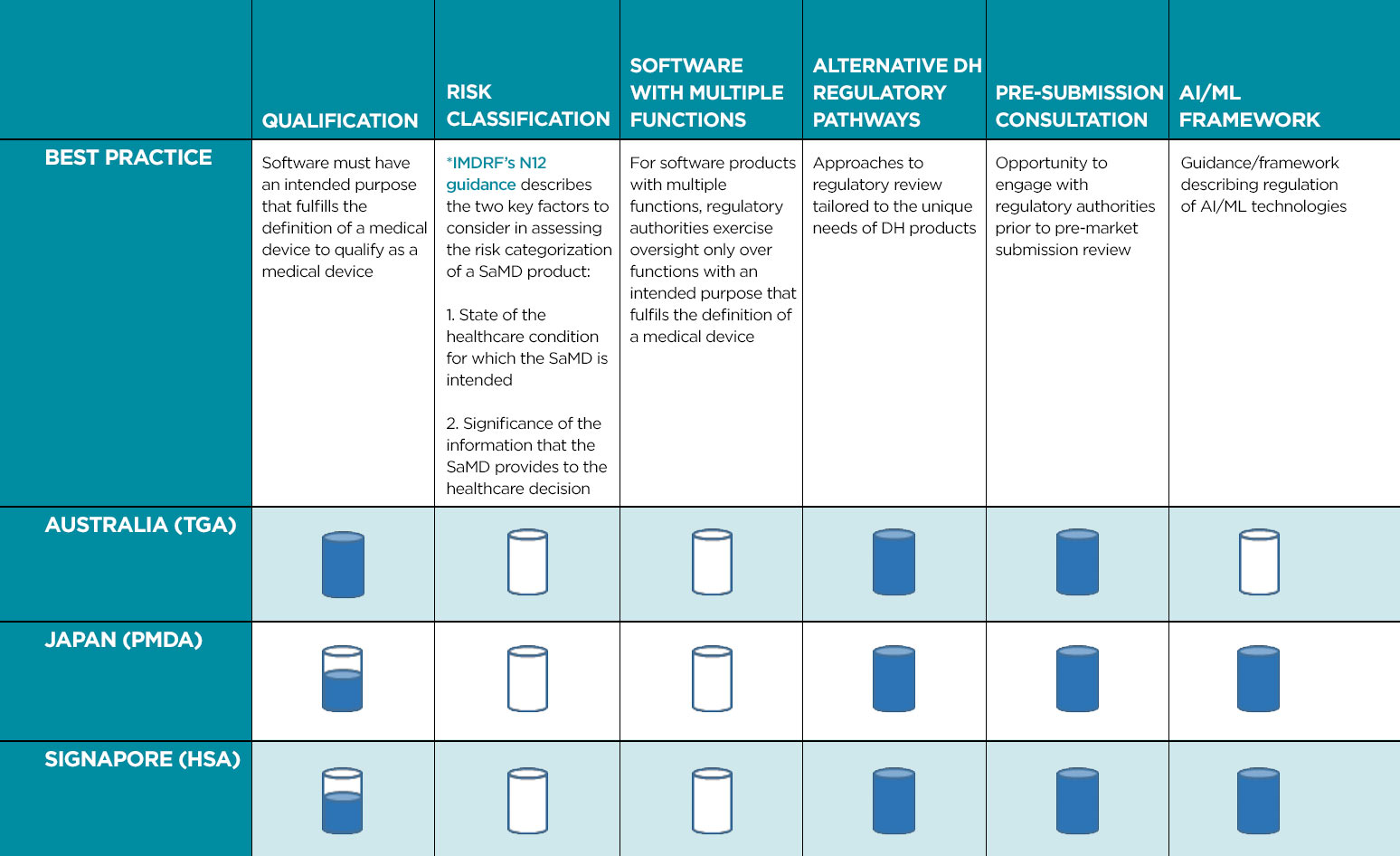
Within a region as diverse as APAC, with different levels of both healthcare provision and regulatory expertise, there is inevitably a wide variation in how DH solutions are brought to market. We reviewed the current approaches applied in three APAC countries (Australia, Japan, and Singapore) that have been active in the regulation of DH. Each one of these countries has a unique regulatory approach, although there are similarities. This review focused on five key areas: Software Qualification, Software as a Medical Device (SaMD) Classification, Alternative Regulatory Pathways, Pre-Submission Consultation, and Frameworks for Regulation of Artificial Intelligence (AI) / Machine Learning (ML).
While it is encouraging to see countries in the APAC region establishing regulatory frameworks specific for DH, it is important that such frameworks converge with global approaches and implement innovative pathways to enable timely delivery of safe and effective DH solutions to the market. Convergence of DH regulation across APAC countries will ensure greater consistency and predictability in regulatory review processes, which, in turn, will enable safe, effective, and innovative DH solutions to reach patients and healthcare professionals in an expeditious manner. Further, implementation of regulatory pathways tailored to the unique needs of DH products will foster innovation that will benefit developers and patients alike. With these concepts in mind, Table 1 provides an overview of best practices for the regulation of DH in APAC.
 Current regulatory framework encompasses recommended best practices.
Current regulatory framework encompasses recommended best practices.
 Some guidelines currently available but further improvements are recommended.
Some guidelines currently available but further improvements are recommended.
 Best practices are not currently adopted.
Best practices are not currently adopted.
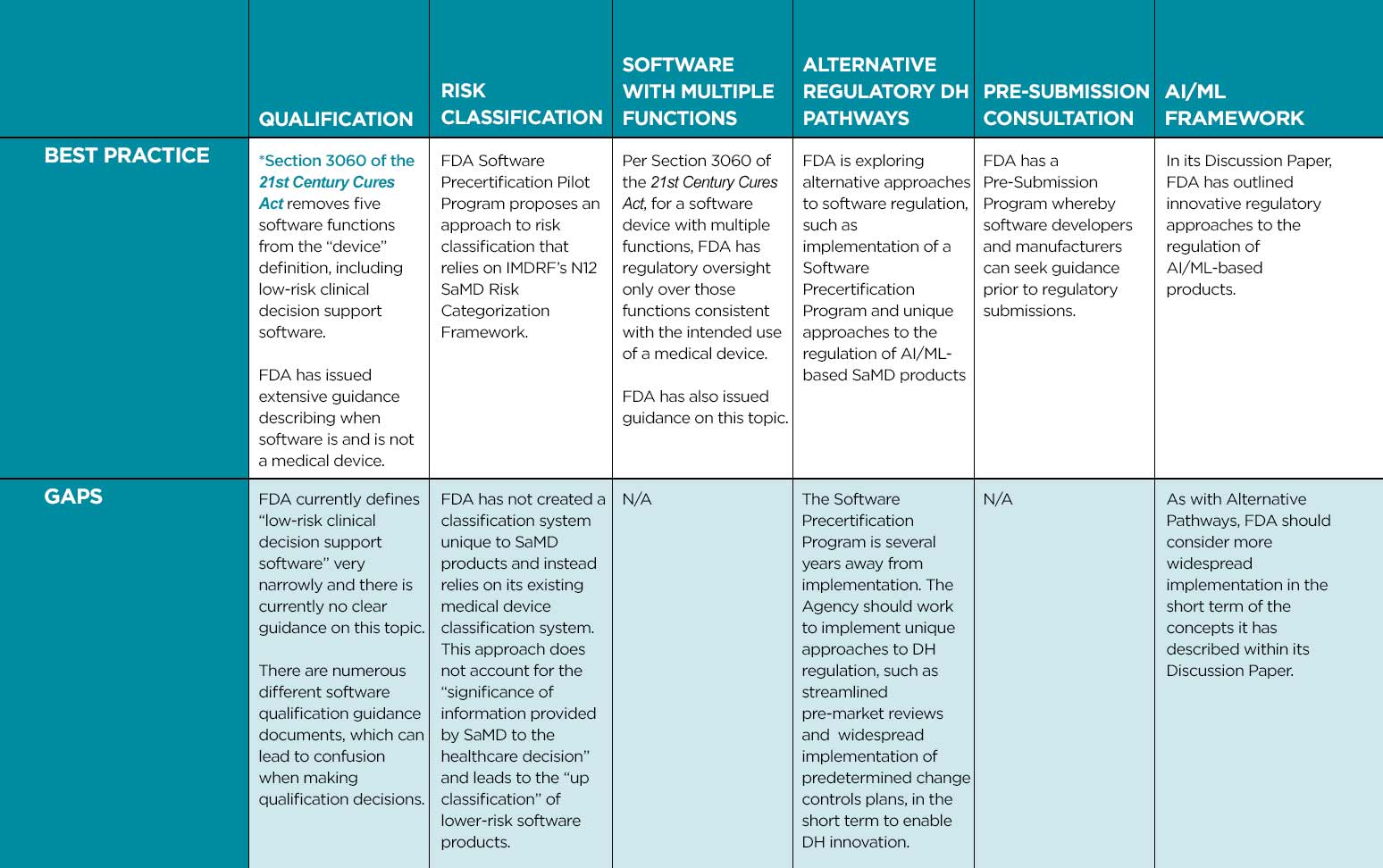
Overview of Digital Health Regulation in the US: Best Practices and Gaps
Best Practices Framework
We outline below best practices that we believe health authorities should apply and areas where fit-for-purpose and risk-based DH regulatory frameworks can be considered.
Fundamental Building Blocks
- Implement a clearly described approach to software qualification (determining when software is SaMD) whereby the health authority has oversight only over those software functions consistent with the intended use of a medical device. This approach should leverage international best practices such as those used in the US, Canada, and Australia.
- Create a SaMD-specific classification approach that does not leverage existing classification schemes for traditional medical devices but is based upon IMDRF’s N12 SaMD Risk Categorization Framework. The “state of healthcare situation or condition” and “the significance of information provided by the SaMD to the healthcare decision” must be considered when making SaMD classification decisions.
- For software products with multiple functions, implement policies that only regulate functions consistent with the intended use of a medical device.
Pathways for Rapid Review of SaMD Products and Their Modifications
- Implement recognition and reliance models which utilize regulatory assessments from comparable overseas regulators when making DH regulatory decisions.
- Streamline regulatory pathways for introducing SaMD products and their modifications, such as developing expedited review pathways and endorsing use of predetermined change control plans.
- Consider unique regulatory approaches, tailored to the iterative nature of SaMD solutions, that leverage artificial intelligence.
Collaboration and Convergence Opportunities in the APAC Region
- Support DH regulatory global convergence through recognition and adoption of internationally recognized guidance documents and standards such as those developed by IMDRF and ISO.
- Collaborate with software developers through Pre-Submission Consultations.
- Partner with industry through industry associations, private-public consortiums, and other fora to share best practices and help the DH regulatory landscape evolve to enable the safe, effective, and timely delivery of innovative solutions benefiting healthcare professionals and patients.
The authors extend their gratitude and appreciation to all members of and contributors to the APACMed Digital Health Regulatory Working Group.
The Asia Pacific Medical Technology Association (APACMed) represents manufacturers and suppliers of medical equipment, devices and in vitro diagnostics, industry associations, and other key stakeholders associated with the medical technology industry in the Asia Pacific region. APACMed’s mission is to improve the standards of care for patients through innovative collaborations among stakeholders to jointly shape the future of healthcare in this region. In 2020, APACMed established its Digital Health Committee to support its members in addressing regional challenges in digital health.

