Rising to the Challenges of Developing Rare Disease Treatments
Cecil Nick
Arlene Hughes
Richard Macaulay
Parexel International, Inc.
rare disease is a disorder affecting a small fraction of the population compared with other prevalent diseases. A significant number of rare diseases are severe, progressive, life-threatening or chronically debilitating
Approximately 7,000 known rare diseases affect an estimated 350 million people worldwide. Most rare diseases are genetic in origin, and a large proportion of rare diseases (65-75 percent) have their onset in childhood. Only five percent of rare diseases have effective treatment; hence, there is a huge unmet need in this area. The US FDA and the European Union (EU) define a drug or biologic intended to treat a rare disease or condition as an orphan drug.
Challenges that clinical trials to develop orphan drugs face include:
- small populations with limited opportunity for study participation and replication of results in larger trials
- heterogeneous manifestation of disease and phenotypic presentations
- poorly understood disease and pathophysiology (on average, a rare disease patient visits seven specialists, and a correct diagnosis can take as long as five to eight years)
- lack of consensus on clinical outcome measures and poorly defined endpoints.
2017 and 2018 brought the greatest number of orphan drug approvals so far. In those two years alone, over 40 percent of all approved drugs received orphan designation from at least one of the major regulatory agencies. FDA granted orphan drug designation to more than 429 unique drugs under development, and approved 46 new orphan drugs, in 2017; in 2018, FDA approved 59 orphan drugs. A number of breakthrough therapies have recently been introduced for diseases that previously had no treatment, including two products for lysosomal storage disorders (Brineura® and Mepsevli®), for spinal muscular atrophy (Spinraza®), the first gene therapy for biallelic mutation-associated retinal dystrophy (Luxturna®), and a novel treatment for Amyotrophic Lateral Sclerosis (Radicava®).
According to Evaluate Market Research, the non-orphan drug market is growing at about six percent annually while the Orphan Drug market is growing at about 11 percent annually. There are a few reasons for this increase. Scientific advances now include the use of precision medicine, leveraging human genomic and biomarker data to improve drug target identification and patient engagement. Additional innovative approaches include use of artificial intelligence, digital technology, and patient-centricity. Regulatory agencies such as the FDA (US), EMEA (EU), and PMDA (Japan) have created incentives attached to orphan drug designation.
EU and US Orphan Drug Regulations
Both US and EU regulatory agencies have initiated programs to encourage development of treatments for orphan diseases, as have other major regulatory bodies such as PMDA in Japan. A key feature of these programs is the creation of a “first past-the-post race” whereby the first drug approved enjoys exclusivity barring market entry of similar compounds for seven years in the US and ten years in the EU. Thus, sponsors must be aware of and capitalize on available incentives and optimize their development programs to ensure that their product is first to market or offers additional benefits over existing approved therapies.
But the definition for a qualifying orphan disease differs between the EU and US. In the EU, an orphan designated product must treat, prevent, or diagnose a life-threatening or chronically debilitating disease involving a condition with an EU prevalence of not more than five in 10,000 persons. In the US, the prevalence cut off is less than 200,000 cases within the US, which is just slightly more than six in 10,000. In Japan, the prevalence is four in 10,000.
One challenge in both EU and US is that such a small patient population means that the product is unlikely to generate enough financial return to justify the investment needed to develop it. In the EU, additional conditions must be met: There must be no satisfactory method of diagnosis, prevention, or treatment of the orphan condition, or the proposed orphan drug must provide significant benefit to those affected, which are not requirements in the US. Developing a successful application for orphan designation can be challenging because evidence must be provided that the above criteria can be met, which may require extensive literature and database review. The challenge does not stop there, particularly in the EU, where the need to prove significant benefit at the time of market approval makes expertise in orphan drug regulatory requirements a necessary component of the development team.
Several incentives and benefits for designated orphan products in the EU and US are summarized below.
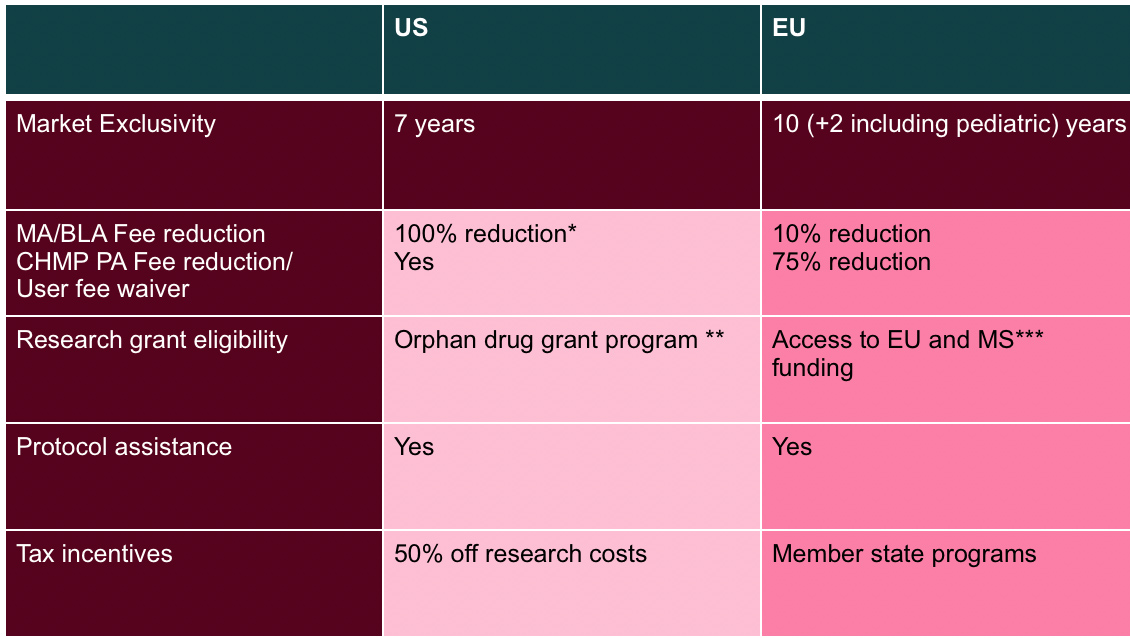
* unless application also includes non-orphan disease/condition
** typically university investigators and small companies
*** MS = Member State
The most critical incentive awarded to an approved orphan designated medicine is market exclusivity, which means that no other marketing approval can be granted for the same therapeutic indication in respect of a product similar to the orphan medicine, unless, compared to that product, such therapy is proven to be safer, more effective, or in some other way to offer what is termed in the EU “significant benefit” and in the US a “major contribution” to patient care.
To maximize the opportunity to be first to market and/or show significant benefit to capture the prize of market exclusivity, an efficient and effective clinical development program is paramount.
Regulatory Requirements for Approval of Medicines Treating Orphan Diseases
It is important to note that an orphan designation does not allow the sponsor a reduced burden of benefit-risk proof compared to non-orphan medicines. However, orphan medicines will often qualify for accelerated regulatory pathways such as accelerated approval, conditional approval, or approval under exceptional circumstances in the EU; and accelerated approval, priority review, and fast track in the US. Orphan medicines may also qualify for support schemes such as the breakthrough therapy designation in the US and PRIME in the EU, another area where regulatory expertise can provide valuable guidance to the orphan drug development team.
Designing a clinical program that will result in regulatory approval is particularly challenging for an orphan medicine because there are unlikely to be disease-specific guidelines or precedence and the choice of the primary endpoint may not be clear. Certainly, development will be simpler if the efficacy of the product is dramatic and easy to measure, and the target condition is life-threatening or seriously debilitating. While the latter may often be true, the former is sadly not.
Designing these clinical programs is further confounded by the limited availability and wide geographic spread of patients. Innovative program design and robust justification of this thinking to regulators once again call for skilled regulatory support.
Since the number of patients is so limited, orphan medicine trials must span a wide geography, requiring regulatory knowledge of global clinical trial and regulatory approval requirements. Since no other satisfactory treatments are likely to exist, there will also be a call for (compassionate) use of the product outside the clinical trial. Regulations governing compassionate use are complex and vary from country to country, even within different EU Member States. Specialized regulatory knowledge is also crucial in this setting.
Challenges in Conducting Trials for Orphan Disease Treatments
Accurate Diagnosis
Diagnosis is the gateway to effective treatment and, in many cases, clinical trial participation. Accurate and early diagnosis of a rare disease can often present a challenge for patients, and in some cases, an accurate diagnosis is not obtained for as long as five years.
Many orphan diseases are known to have a specific causal gene. In some orphan diseases, affected family members are instrumental in directing physicians to conduct rapid targeted testing. In families that carry known and heritable rare disease genes, prenatal, newborn, and/or early childhood screening can drive early diagnosis. Often, preimplantation genetic testing is also considered in families with a known causal genetic mutation.
Continued advancement of genetic testing technologies such as next-generation sequencing can potentially shorten the time to diagnosis in the future. As their cost decreases, tests will be run more routinely for patients with a suspected genetically-driven rare disease, so long as analysis methods are in place to interpret their results.
In most cases, diagnostic laboratories perform the test and produce clinically translatable, reliable results that can have immediate impact on patients and their families. Clinical Laboratory Improvement Amendments (CLIA) and/or FDA quality system regulation (QSR) requirements may be necessary if selection of the appropriate treatment relies on a genetic test.
Patients may present with symptoms that a physician may not readily recognize as associated with or caused by a rare disease. In these cases, broad genetic testing may look for genetic variants that may be responsible for the disease manifestation. Ensuring the patient is either connected with a clinical geneticist and/or physician who is comfortable with navigating the challenges of genetic data interpretation is always helpful. Patient advocacy groups and social media are also providing information and knowledge to patients and their families that can improve diagnosis.
Endpoint Selection
In developing therapeutics for orphan diseases, it is critical to ensure that the clinical study endpoints will measure how the drug impacts the patient’s disease progression. To differentiate a drug effect from the standard course of disease progression, clinical study and endpoint design must appropriately reflect regulatory requirements and potential payer considerations.
Sponsors are advised to work with regulatory agencies to agree on efficacy endpoints before the clinical study. FDA hosts disease-specific fora to understand and discuss which endpoints are important from a patient or caregiver’s perspective; these endpoints can also include validated patient-reported outcomes (PROs) that incorporate the patient’s voice.
Patient Availability and Recruitment
Finding eligible patients for clinical trials in orphan medicines represents a major challenge. Not only is the target population difficult to identify but finding investigators who treat these patients is also complex. These challenges may represent a risk to recruitment and, as a result, require a tailored feasibility approach to mitigate risk in each rare disease study.
Effective recruitment in rare disease indications requires understanding of the relevant disease and the patient profile. A few steps are helpful here, such as liaising with medical experts and obtaining up to date information on the rare disease, including clinical presentation, epidemiology, and treatment, all of which contribute to better understanding the patient profile. It is also necessary to define how to engage and include eligible patients into the study and/or how to raise awareness of the study among patients. Collaboration with patient advocacy organizations can provide essential patient education and awareness, and these groups are also often able to identify suitable study investigators.
With the potential wide geographic spread of the patient population, patients may need to travel long distances to get to the study site of an orphan disease trial. Although travel reimbursement could mitigate this risk, companies should also investigate innovative solutions and technologies that collect data from patients at home and reduce the need for site visits. While some technological innovations have been applied to clinical trials for many years (such as the collection of electronic patient-reported outcome assessments) sponsors are now considering wearable devices as new ways of both engaging with patients and collecting their data. For example, patients can now take blood pressure measurements at home in a platform automatically integrated to the site’s electronic data capture system, moving the patient-site interaction outside the clinic and inside the home.
Other methods can alleviate the burden of patient access to the study site, eventually increasing the chance of successful recruitment. For instance, in pediatric neuromuscular rare diseases, use of transportation assistance services and remote study visits can improve recruitment and retention rates. These methods are useful to reduce the burden of participation for patients who live far away from site.
When defining what additional support can be provided to patients, trial teams should also consider the additional site staff burden. Study coordinators are involved in providing support for patient travel and have positive feedback on concierge services that simplify travel and related reimbursement, so the site can focus on data quality.
Finding the Right Sites
Rare disease studies often require participation from numerous study sites across many regions. Identifying appropriate investigators and sites is complex, as sites with access to the target patient population may not necessarily have the most experienced site staff. In these instances, additional training and support is needed to maintain study integrity. Clinical assessments and equipment may introduce variability across regions, which makes standardization of procedures necessary. Certain patient populations such as adolescents will have contact with and data in sites with both pediatric and adult populations.
Using fewer but more specialized study sites is a reasonable strategy, as well as leveraging knowledge from key opinion leaders based about local sites and standards of care.
Insufficient Data
Wearable devices offer a mechanism for additional data capture in the real world. According to a recent Industry Standard Research report, activity trackers are included in 21 percent of trials using mHealth. By selecting the right device, it is possible to combine data on activity or mobility, on sleep quality and heart rate, measured continuously over a long period of time. Such data points can be used to demonstrate a drug’s positive impact on patients’ well-being and could be used to complement (and in some cases replace) subjective PROs.
While collecting data in the context of the patient’s home, companies should be mindful that the responsibility to be compliant with the protocol lies with the patients and their ability to collect data/samples at the right time. It is recommended that any patient application is configured to meet a protocol’s specific needs, using smartphone notifications on days/times when patients must take measurements or collect PROs. With the right level of reminders and by selecting wearable devices carefully to minimize patient burden, companies can positively impact recruitment and retention and amass additional data that may increase understanding of the natural course of the disease (in placebo treated patients).
Commercialization
Making a therapy accessible to most patients requires not only regulatory marketing authorization but market access via private or public reimbursement. However, reimbursement of orphan products may be challenging: Many products receive marketing authorization with a less than typically comprehensive data package, but at the same time demand high per-patient prices due to low sales volume caused by the low prevalence of the rare disease.
Orphan designations are primarily programs for regulatory incentives, not reimbursement. Although some markets do have specific incentives for the reimbursement of orphan medicines, many do not. Significantly, since 2011, every new pharmaceutical product in Germany must demonstrate additional benefit to the Federal Joint Committee (Gemeinsamer Bundesausschuss, or G-BA) to claim an additional reimbursement benefit from the national statutory health insurance (SHI). However, for drugs approved as an EMA orphan medicine, such additional benefit is assumed proven by its marketing authorization, and the benefit assessment becomes necessary only after costs to the SHI exceed €50M per year.
In many major markets, pricing and reimbursement of new therapies is based on an assessment by a national health technology assessment (HTA) body, for which economic value is typically a key consideration. However, HTA assessments take time and often may not produce favorable outcomes. Research on the reimbursement consequences of European orphan designations revealed that only a small minority of drugs for orphan diseases in England and Scotland (nine and 12 percent respectively) received full recommendations for their licensed indication(s). In France, only 39 percent of assessed drugs received a positive Improvement of Medical Benefit assessment rating (Amélioration du Service Médical Rendu, ASMR) which enables better access (price notification instead of negotiation) and comparative pricing with EU counterparts. In Germany, all orphan drugs received automatic additional benefit post-regulatory approval: G-BA outcomes were all categorized as positive because orphan medicines have special status in early benefit assessment, with automatic non-quantifiable benefit, provided conditions are met. Despite the average 15-month delay, these drugs are available under free pricing before the G-BA final resolution.
These challenges may be magnified in the future by new transformative therapies for orphan indications (e.g., CAR-T and gene therapies) which offer substantial and potentially curative clinical benefits from single treatments. These therapies will likely receive expedited marketing authorization through expedited regulatory approval pathways based upon promising but very early clinical data. Appropriate reimbursement of these potentially very high cost therapies based upon such immature data will be even more challenging under traditional payer models. Further, their single treatment curative nature means that the entire cost of treatment must be paid up front. Appropriate pricing and access to these therapies–which have been approved under expedited regulatory pathways, where data has indicated potential for curative benefits, but where considerable uncertainty remains–will necessitate increased use of alternative reimbursement mechanisms.
Increasingly, many payers have implemented innovative contracting arrangements such as performance-based reimbursement, indication-specific pricing, dynamic pricing, and budget caps. The need for even more innovative approaches, such as leasing, has also been discussed. Many of these systems have broad conceptual appeal but are associated with increased management costs, and there are notable examples of implementation failure. Instead of coalescing on a single reimbursement system, it likely that we will see a wide variety (and combinations) of different mechanisms put in place by and between different payers to manage these therapy classes based upon the therapy area, the precise function of that payer, and their collective iterative experiences.
Conclusion
Challenges in conducting clinical trials in rare and orphan diseases can be overcome with careful planning that incorporates regulatory, clinical, scientific and market access strategy. The patient journey and needs must be understood in order to increase the chance of success. As the number of effective treatments for rare diseases grows, industry will need to adapt to changing regulations, technologies, and reimbursement landscapes, to meet the needs of rare disease patients.
References available upon request.

